Treatment Options
Apart from intestinal disorders, changes in dietary patterns and their effects on gut microbiota are implicated in disorders of other organs such as the lungs (1,2). A diverse and healthy intestinal microbiota supports immune functions that are critical for maintaining homeostasis in the lungs. Nutrition can also affect the immune response and the composition of the respiratory tract microbiota (3). Research emphasises the importance of diet and composition of gut microbiota in determining lung immune response (4).
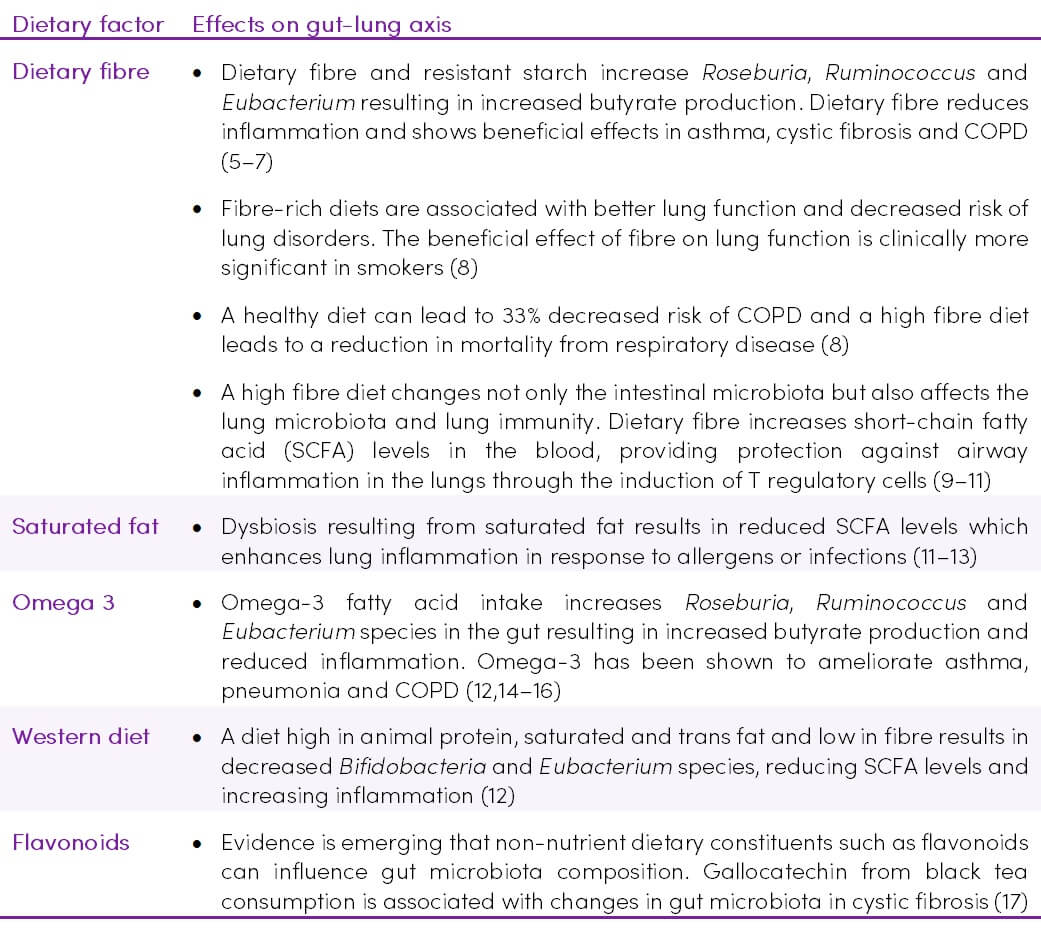
Table 1. Nutritional factors affecting the gut-lung axis
Pre and probiotics
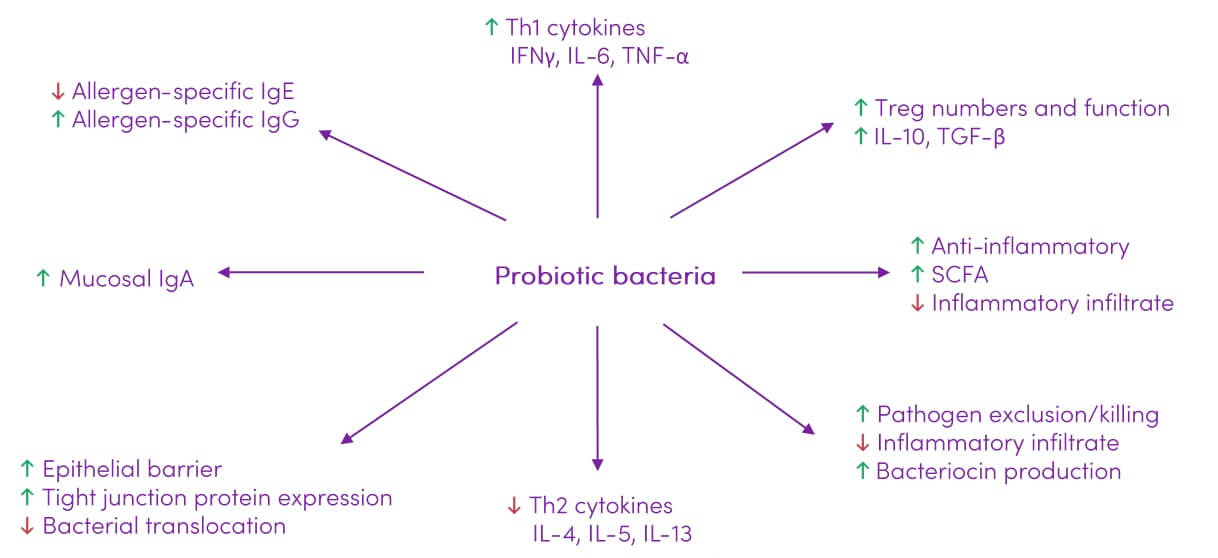
Probiotics show potential in modulating the immune response which can prove beneficial in handling a variety of lung diseases. Probiotics have been shown to improve inflammatory conditions and enhance gut barrier function, thereby preventing antigens such as lipopolysaccharide (LPS) from leaking through. The beneficial roles of probiotics make them potential candidates for the treatment of inflammatory diseases such as allergies, COPD and asthma (4).
Clinical trials to date have yielded inconsistent results, making it difficult to provide definitive treatment options for modulating the gut-lung axis with the use of probiotics. Although experimental research demonstrates that probiotic species (B. lactis, Saccharomyces cerevisiae, Clostridium butyricum, Lactobacilli) inhibit allergic airway inflammation, most clinical data suggests they are not sufficient to reduce asthma-related events (11). Beneficial effects of probiotics on the aspects of human immune response that influence allergy and inflammation are outlined in Table 2.
Table 2. Modulating the gut-lung axis with pre and probiotics

Mechanisms of action
The gut microbiota affects distal organs either by aspiration or by the production of metabolites that bring about changes when they reach other organs (34,35). Bacteria from the gut can travel to the lung through aspiration of vomit or oesophageal reflux. During gut microbiome dysbiosis, disturbed epithelial integrity may enable bacteria and their components and metabolites to enter the circulation causing systemic inflammation (36).
Gut microbiota influences lung immune response
Evidence is increasing in support of a ‘common mucosal response’, which states that the effects of gut microbiota on the mucosal immunity may have an influence on the immune responses at distal mucosal sites, including in the lung. Gut bacterial cells, as well as their metabolites, might stimulate the immune response in the lungs (37). Figure 1 shows some of the mechanisms of immune system programming by the microbiota.
Figure 1. Interaction between the immune system and the intestinal microbiota (38) CC BY 4.0
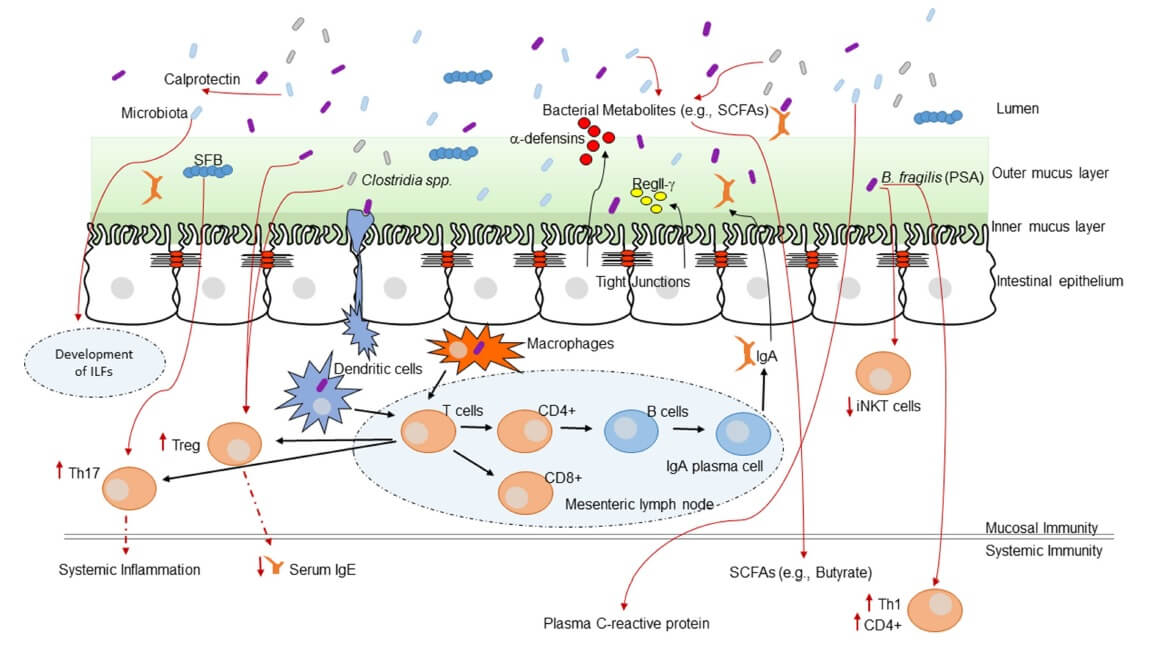
- Microbial metabolites, such as SCFAs induce anti-inflammatory responses and decrease inflammation through the activation of G protein-coupled receptors (11). Microbial metabolites enter systemic circulation via the intestinal lymphatic system and can modulate lung immune response (9,11,35,39)
- Virulence factors from pathogenic bacteria, such as Helicobacter pylori or Bacteroides fragilis, can downregulate host immune responses (36)
- Structural components from commensal bacteria can influence inflammatory responses through the activation of pattern recognition receptors (36)
- The lymph and/or bloodstream acts as a link between the gut (where primary sensitization occurs) and the affected site on the lung (40)
- The immune cells which reside in the intestinal lamina propria and mesenteric lymph nodes can neutralize most of the translocating bacteria. The surviving bacteria as well as the fragments of dead bacteria travel from the mesenteric lymphatic system and enter systemic circulation (34). Following sepsis or acute lung injury, in the presence of increased systemic inflammation the lung microbiome shifts to gain gut microbial species (41). Although the mechanism for the change in lung microbiome is not known, gut microbes may be introduced into the circulation and penetrate the inflamed lung or enter the lung via aspiration
- Histamine is an important immunomodulator influencing both the innate and adaptive immune system. Bacterial strains can secrete histamine, as well as express histidine decarboxylase (HDC) enzyme, which is responsible for catalysing the decarboxylation of histidine to histamine. Histamine secretion from bacteria within the gut can have immunological consequences at distant mucosal sites, such as within the lung (42)
- Gut microbiota promote and maintain the differentiation of Treg cells for maintaining the homeostasis of the intestinal system and inhibiting excessive inflammatory responses (43). T cell receptor signalling may be the primary pathway in the communication between gut and lung (9,39,44)
- Antigen exposure in the gut lumen can induce specific IgA-containing cells in the blood. The role that IgA plays in airway protection is unquestioned (40)
- Diet and probiotics regulate lung inflammation through the induction of metabolites and Tregs (11)
Probiotics
Probiotics show potential in modulating the immune response (Figure 2) which can impact a variety of lung diseases.
Figure 2. Immune modulating effects of probiotics. Adapted from (25) under CC BY 3.0
Bi-directional communication
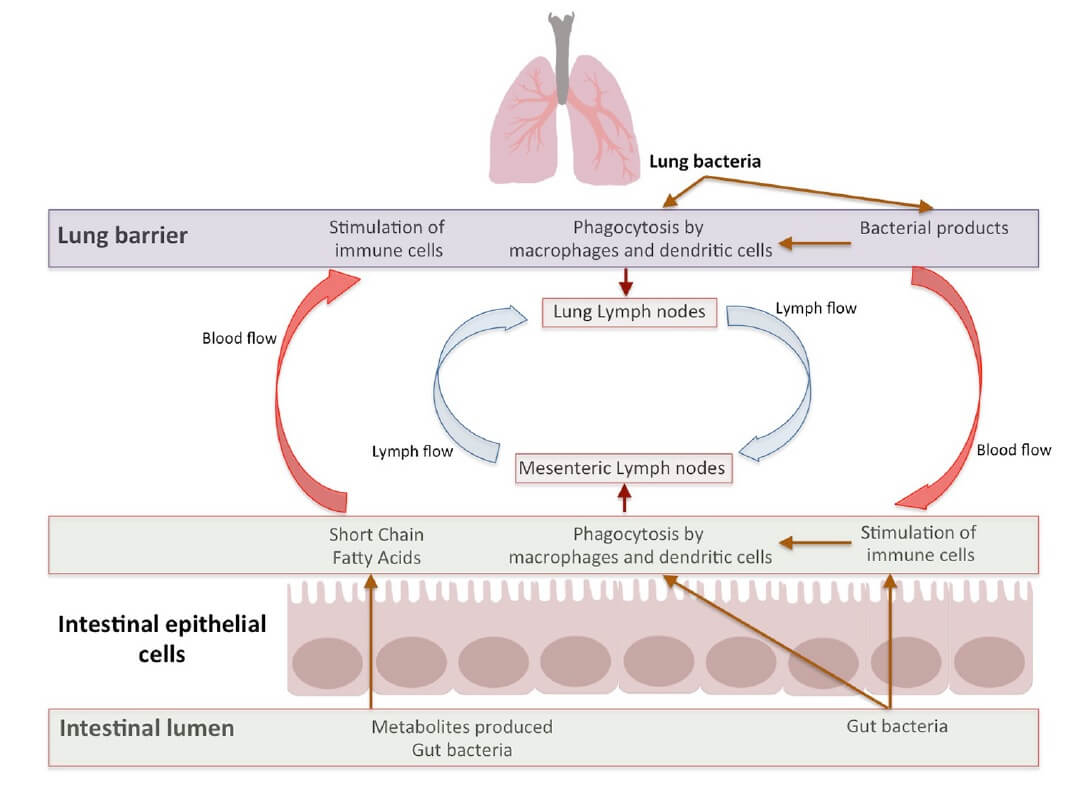
- The gut-lung axis is a bidirectional loop which is stimulated by the changes in gut or lung immunity (34,36). Intestinal microbiota influences lung immunity, and lung inflammation influences intestinal microbiota and causes disease. Apart from metabolites, the immune cells, which are induced by a multitude of antigens, move through lymphatic duct between both the gut and the lung leading to modulation of immune response in both organs (Figure 3) (43)
- Signals from the inflamed lung direct alterations in the intestinal bacterial community structure which further support inflammation in the lung (45)
- Lung microbiota and its metabolites move to the intestines through the bloodstream and vice versa (40). In this way, they may pass through the liver and accordingly activate immune cells such as neutrophils and macrophages (46)
Figure 3. Bidirectional gut-lung axis communication (4) CC BY 4.0
Takeaway on the gut-lung axis
- The communications between microbiota and the immune system are bidirectional and complex and we are still far from comprehensively understanding how a well-balanced immune system develops (40)
- The beneficial effect of pre and probiotics have been observed in several lung ailments, although the results are inconsistent, possibly due to differences in strains and dosages. These inconsistencies in study results make it difficult to conclude whether probiotics have beneficial roles in patients with respiratory disorders (4)
- Dysbiosis in gut microbiota and metabolites such as SCFAs significantly influence the systemic immunity of not only the GI system but also other organs through lymphatic and circulatory systems. The common mucosal system ensures that antigens introduced in the gut are capable of eliciting an immune response in the lungs (4)
- Changes in lung microbiota results in effects on the intestine, however a complete understanding of the system requires further studies (4)
- The influence of intestinal microbiota on exacerbations that are distinct from disease aetiology remains an understudied area (11)