Treatment Options
Treatment choices need to be determined based on which heavy metal needs to be removed (1).
General treatment options
Probiotics
Lactobacilli probiotics are ideal as an adjunct tool to prevent/reduce heavy-metal toxicity and prevent absorption of metals into the human body. Lactobacilli have the ability to bind and sequester metals (2).
Minerals
Selenium, iron, copper and zinc (1):
- Compete with intestinal absorption of metals
- Decrease the replacement of essential ions and formation of insoluble metal-mineral complexes
- Induce the production of metal binding proteins (metallothioneins)
Zinc, calcium and vitamin E
Supplementation with zinc, calcium and vitamin E has a broad benefit and low risk in toxic metal treatment (1200 mg/day calcium; 13mg/day zinc sulphate/gluconate; 19mg (28.4 IU) vitamin E) (3).
- Provide substrates that induce metallothionein synthesis
- Compete with the toxic metal in question for a variety of carrier proteins, cellular enzymes and cellular structural proteins
- Provide antioxidant protection
Antioxidants
SAMe, lipoic acid, glutathione, selenium, zinc, NAC, methionine, cysteine, alpha-tocopherol, and ascorbic acid have specific roles in the metabolism and excretion of heavy metals (4). Glutathione has the ability to form complexes with different metals (1).
Arsenic
- Zinc improves excretion of metals such as lead and arsenic from the body (1)
- Folate supplementation (400-800µg/day of folic acid for 12-24 weeks) lowers blood arsenic levels by facilitating urinary excretion of arsenic (5,6,7)
- Increase intake of folic acid, methionine, choline, and vitamins B6and B12
Mercury
- Fruit consumption may have a protective effect against methylmercury exposure. Soluble dietary fibre and prebiotic nutrients potentially impact methylmercury metabolism in the GIT (9,10)
- Consumption of tomato products has an inverse relationship to blood mercury concentrations (11)
- Glutathione binds with mercury in the liver, reducing the mercury burden on the body and increasing excretion by the kidneys. Glutathione also protects the kidneys from mercury induced cellular injury (1)
Lead
- NAC (200 to 800 mg/day) can help to normalise antioxidant activities in blood cells and normalise homocysteine levels in chronic lead poisoning (12,13)
- Allium sativum (garlic) reduces blood lead levels in chronic lead poisoning. Dried powdered garlic (400 mg; equivalent to 1200 mg allicin or 2 g fresh garlic) administrated 3 times a day for 4 weeks reduces the neurological effects of lead such as irritability and headache (14)
- Coriandrum sativum (coriander) extract decreases blood lead levels in children by potentially causing renal clearance of lead (15)
- Calcium supplementation (1200mg/day) or milk/dairy intake may decrease the uptake of lead in the gastrointestinal tract (16)and protect against the effects of lead in pregnant women (17,18)
- Antioxidants
- Vitamin E 400 IU and vitamin C 1 g daily for one year can reduce the oxidative damage induced by lead intoxication as well as increase the total antioxidant capacity (19)
- Vitamins B1, B6, C and E provide an elevated therapeutic impact in lead toxicity when administered with thiol chelators DSMSA and MiADMSA (20)
- Iron counteracts the toxic effects of lead. Iron deficiency has been found to be associated with exacerbation of the toxicity of lead (1)
- Zinc improves excretion of metals such as lead and arsenic from the body (1)
- Metallothionein content of foods may affect bioavailability and metabolism of lead. Metallothionein binds lead with high efficiency, however, it is still unclear how it acts to transport or detoxify the metal ion (21,22). Minerals/foods that increase the levels of metallothionein includecopper, zinc, selenium, cysteine, and histidine; hops, pomegranate, watercress, cruciferous vegetables (23,24,25), quercetin and Cordyceps sinensis
Cadmium
- Probiotic treatment can be used as one of the strategies for prevention of cadmium toxicity, in parallel with chelation, antioxidant, and anti-inflammatory therapy (26)
- Iron deficiency is associated with increased cadmium body burden (27,28)
Aluminium
Silicon-rich mineral water (35 mg/L as silicic acid) can increased aluminium excretion from the body. Regular consumption of 1-1.5 litres per day is recommended (29,30).
Mechanisms of action
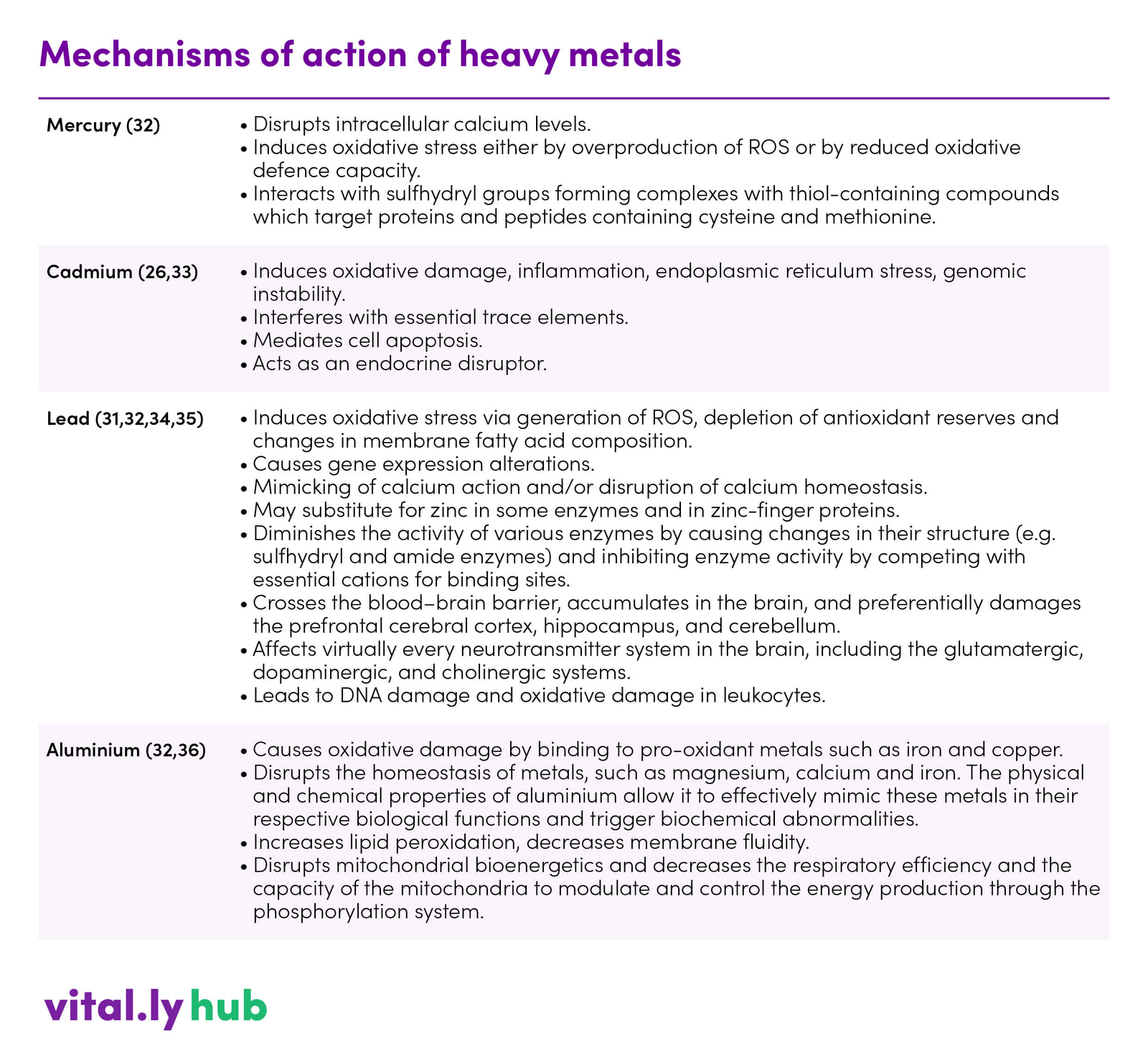
The general mechanisms involved in heavy metal induced toxicity include (1,31):
- Production of reactive oxygen species resulting oxidative damage
- Accumulation in the body, where they are substituted for essential elements. For example, calcium substituted by lead, zinc substituted by cadmium and the majority of trace elements are substituted by aluminium
- Stored heavy metals destroy major metabolic processes of the body
- They influence the activity of various hormones and the function of essential enzymes
- Susceptibility of body toward infection is increased as a result of alterations in carbohydrate, protein and lipid metabolism
All of the above mentioned mechanisms ultimately alter the synthesis of neurotransmitters and their use in body thus altering central nervous system functions.
Table 1. Mechanisms of action of heavy metals
Cautions
- Treatment options are specific to individual heavy metals. For example, selenium supplementation is associated with reversing the effect of mercury, lead and arsenic, however, high levels of selenium enhance the toxicity of lead, so it is not be an appropriate choice of therapy in all cases (1)
- The form of the toxic metal exposure needs to be taken into account (inorganic vs. organometallic vs. metallic) for some metals. For example, methylmercury is well removed by some substances that do not remove inorganic sources of mercury (3)
- The competition between metals for various carriers and other proteins requires a sufficient dose of the essential elements (e.g., calcium, zinc) to displace the toxic metal. However, high dosages could be wasteful or toxic (for example, selenium) (3)
- Displacing a metal from one tissue may increase its concentration in another. For example, displacing methylmercury from the liver may make it more available for the brain (3)
- Most, if not all metals, once displaced from a tissue will need to be excreted by the kidney, resulting in a change in kidney function in the short term. Caution is warranted in cases of renal insufficiency (3)
- Administering a combination formula requires care to ensure that interactions do not occur rendering one of more of the compounds bio-unavailable. For example, administration of zinc simultaneously with a thiol chelator would produce an inert and bio-unavailable zinc-chelator complex (3)
Common markers
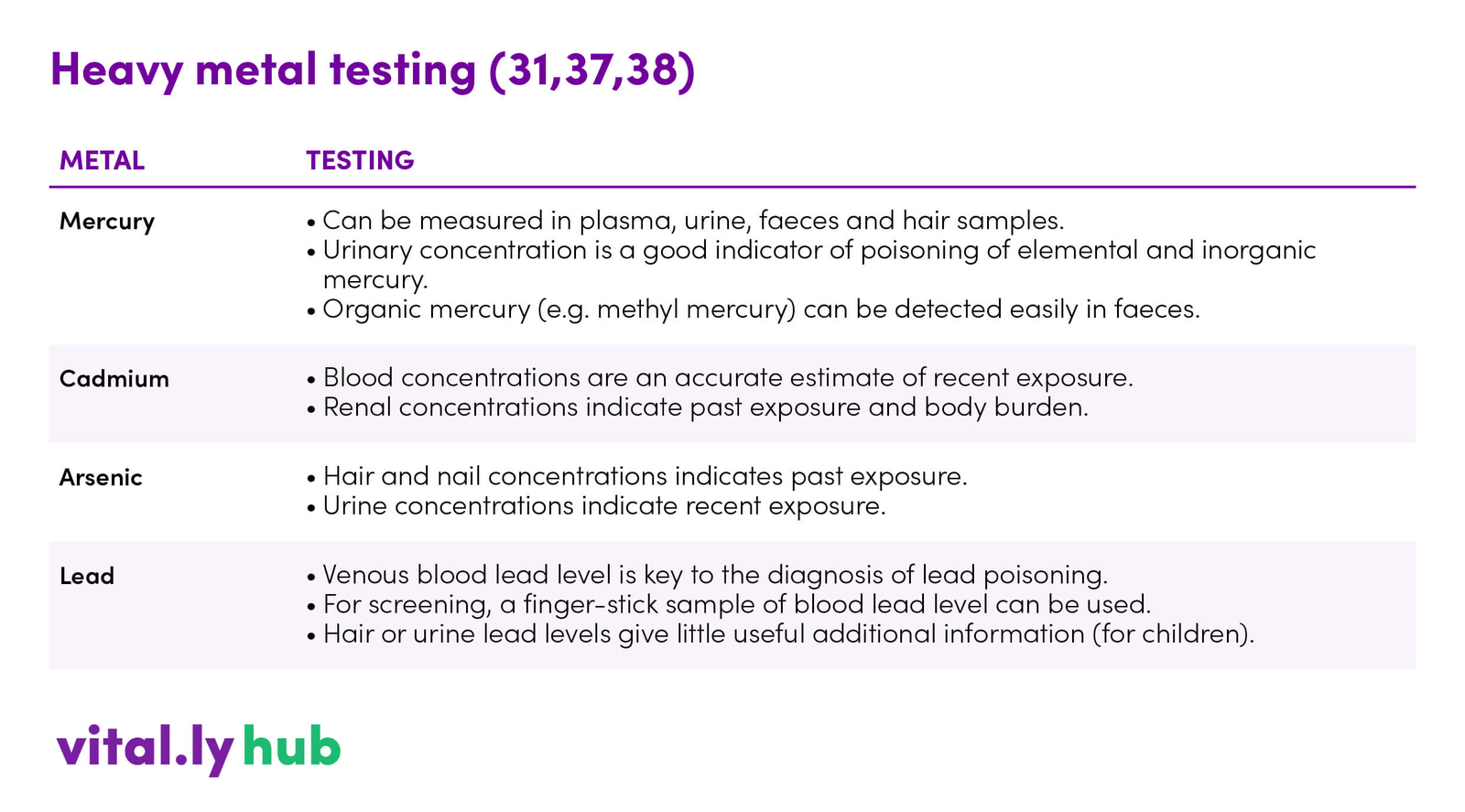
A hair tissue mineral analysis can be a good starting point. More specific testing options are shown in table 2.
Table 2. Heavy metal testing (31,37,38)
Takeaway on Heavy Metals
- Heavy metals are common environmental health risks with many adverse health effects
- Prevention is always the best medicine
- It is important to identify and reduce sources of heavy metal exposure
- Therapy needs to be metal specific
- Malnourished or essential metal deficient patients are at a distinct disadvantage for dealing with metal intoxication (3)
- All patients would benefit from a sound nutritional status
A separate summary of heavy metal treatment options is available here





