| 1 | Andrade VM, Aschner M, Marreilha dos Santos AP. Neurotoxicity of Metal Mixtures. In: Aschner M, Costa LG, editors. Neurotoxicity of Metals [Internet]. Cham: Springer International Publishing; 2017. p. 227–65. |
| 2 | Badr FM, El-Habit O. Heavy Metal Toxicity Affecting Fertility and Reproduction of Males. In: Bioenvironmental Issues Affecting Men’s Reproductive and Sexual Health [Internet]. Elsevier; 2018. p. 293–304. |
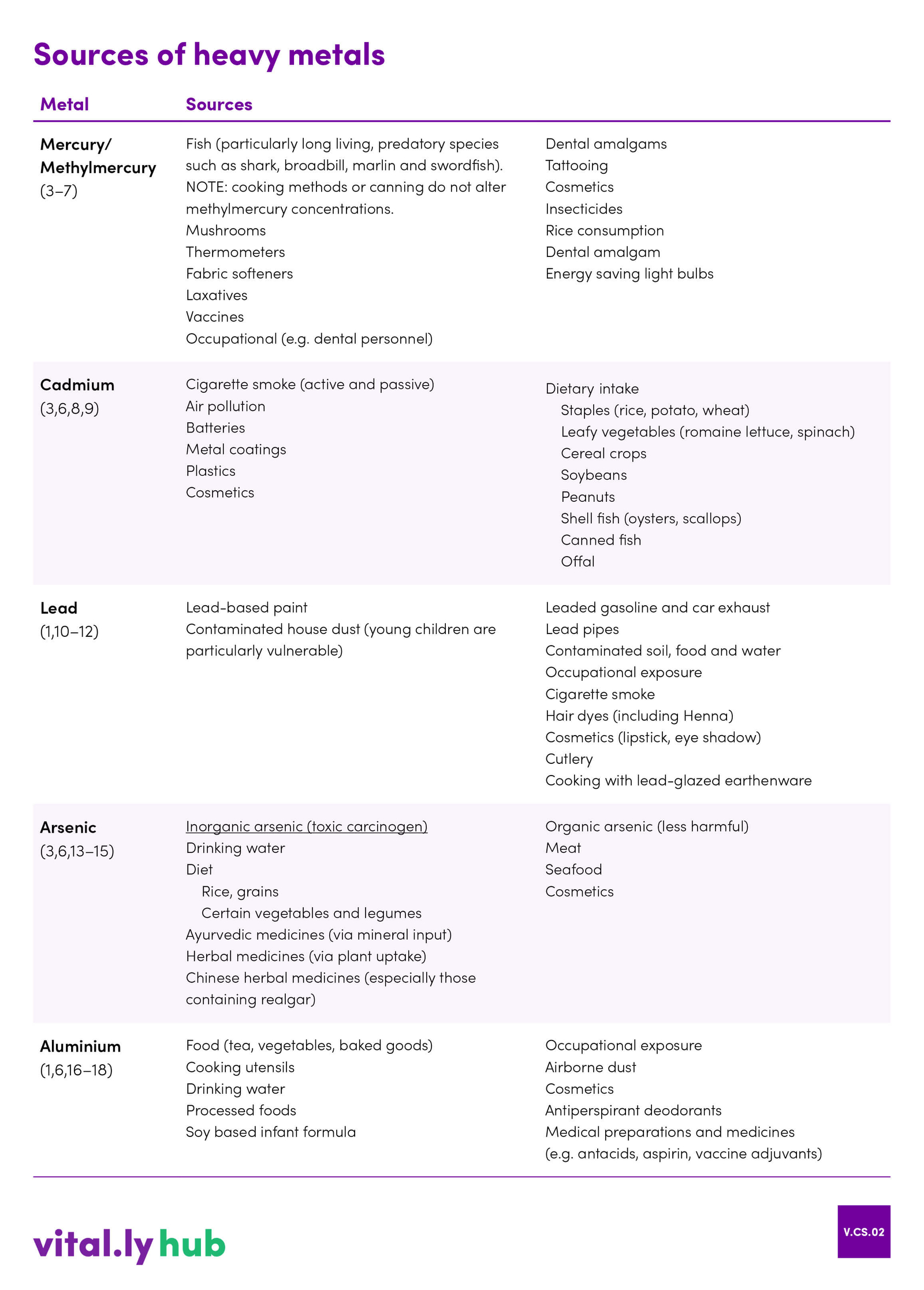
| 3 | Chiocchetti G, Jadán-Piedra C, Vélez D, Devesa V. Metal(loid) contamination in seafood products. Critical Reviews in Food Science and Nutrition. 2017 Nov 22;57(17):3715–28. |
| 4 | Aaseth J, Hilt B, Bjørklund G. Mercury exposure and health impacts in dental personnel. Environmental Research. 2018 Jul;164:65–9. |
| 5 | Bjørklund G, Dadar M, Mutter J, Aaseth J. The toxicology of mercury: Current research and emerging trends. Environmental Research. 2017 Nov;159:545–54. |
| 6 | Borowska S, Brzóska MM. Metals in cosmetics: implications for human health: Metals in cosmetics. Journal of Applied Toxicology. 2015 Jun;35(6):551–72. |
| 7 | Ha E, Basu N, Bose-O’Reilly S, Dórea JG, McSorley E, Sakamoto M, et al. Current progress on understanding the impact of mercury on human health. Environmental Research. 2017 Jan;152:419–33. |
| 8 | Gomes de Moura CF, Ribeiro DA. Are food compounds able to modulate noxious activities induced by cadmium exposure? Critical Reviews in Food Science and Nutrition. 2017 Feb 11;57(3):632–6. |
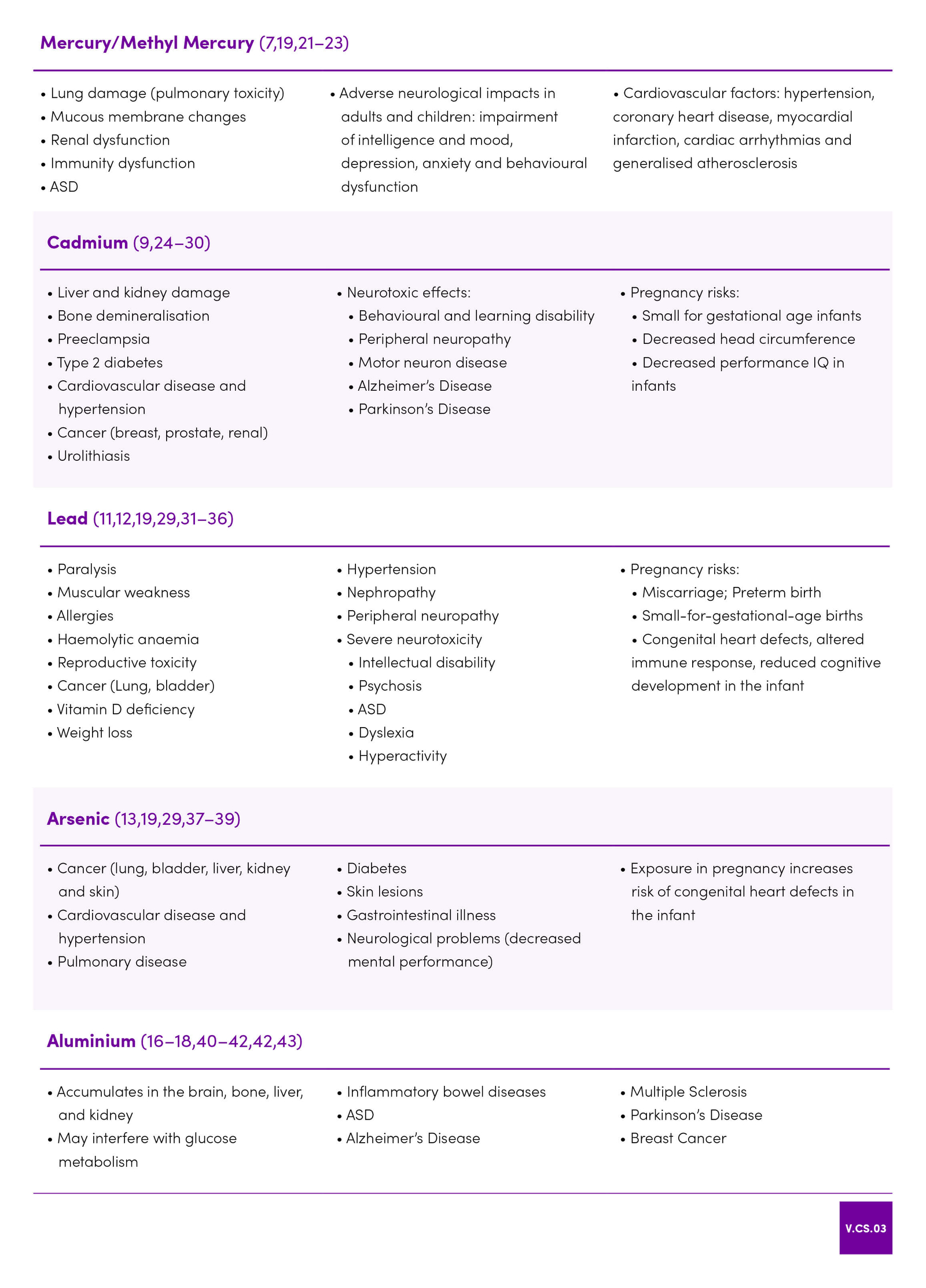
| 9 | Jacobo-Estrada T, Santoyo-Sánchez M, Thévenod F, Barbier O. Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. Int J Mol Sci [Internet]. 2017 Jul 22;18(7). |
| 10 | Kaličanin B, Velimirović D. A Study of the Possible Harmful Effects of Cosmetic Beauty Products on Human Health. Biological Trace Element Research. 2016 Apr;170(2):476–84 |
| 11 | Pohl HR, Ingber SZ, Abadin HG. 13. Historical View on Lead: Guidelines and Regulations. In: Sigel A, Sigel H, Sigel RKO, editors. Lead – Its Effects on Environment and Health [Internet]. Berlin, Boston: De Gruyter; 2017. |
| 12 | Sachdeva C, Thakur K, Sharma A, Sharma KK. Lead: Tiny but Mighty Poison. Indian Journal of Clinical Biochemistry. 2018 Apr;33(2):132–46. |
| 13 | Bolan S, Kunhikrishnan A, Chowdhury S, Seshadri B, Naidu R, Ok YS. Comparative analysis of speciation and bioaccessibility of arsenic in rice grains and complementary medicines. Chemosphere. 2017 Sep;182:433–40. |
| 14 | Ettinger AS, Arbuckle TE, Fisher M, Liang CL, Davis K, Cirtiu C-M, et al. Arsenic levels among pregnant women and newborns in Canada: Results from the Maternal-Infant Research on Environmental Chemicals (MIREC) cohort. Environmental Research. 2017 Feb;153:8–16. |
| 15 | Liu L, Zhang Y, Yun Z, He B, Zhang Q, Hu L, et al. Speciation and bioaccessibility of arsenic in traditional Chinese medicines and assessment of its potential health risk. Science of The Total Environment. 2018 Apr;619–620:1088–97. |
| 16 | Bondy SC. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. NeuroToxicology. 2016 Jan;52:222–9. |
| 17 | Colomina MT, Peris-Sampedro F. Aluminum and Alzheimer’s Disease. In: Aschner M, Costa LG, editors. Neurotoxicity of Metals [Internet]. Cham: Springer International Publishing; 2017. p. 183–97. |
| 18 | Vignal C, Desreumaux P, Body-Malapel M. Gut: An underestimated target organ for Aluminum. Morphologie. 2016 Jun;100(329):75–84. |
| 19 | Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int J Mol Sci. 2015 Dec 10;16(12):29592–630. |
| 20 | Saghazadeh A, Rezaei N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017 Oct;79:340–68. |
| 21 | Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury Exposure and Heart Diseases. Int J Environ Res Public Health [Internet]. 2017 Jan;14(1). |
| 22 | Jafari T, Rostampour N, Fallah AA, Hesami A. The association between mercury levels and autism spectrum disorders: A systematic review and meta-analysis. Journal of Trace Elements in Medicine and Biology. 2017 Dec;44:289–97. |
| 23 | Oulhote Y, Shamim Z, Kielsen K, Weihe P, Grandjean P, Ryder LP, et al. Children’s white blood cell counts in relation to developmental exposures to methylmercury and persistent organic pollutants. Reproductive Toxicology. 2017 Mar;68:207–14. |
| 24 | Everson TM, Armstrong DA, Jackson BP, Green BB, Karagas MR, Marsit CJ. Maternal cadmium, placental PCDHAC1 , and fetal development. Reproductive Toxicology. 2016 Oct;65:263–71. |
| 25 | Guo Z-L, Wang J-Y, Gong L-L, Gan S, Gu C-M, Wang S-S. Association between cadmium exposure and urolithiasis risk. Medicine (Baltimore) [Internet]. 2018 Jan 5;97(1) |
| 26 | Jablonska E, Socha K, Reszka E, Wieczorek E, Skokowski J, Kalinowski L, et al. Cadmium, arsenic, selenium and iron– Implications for tumor progression in breast cancer. Environmental Toxicology and Pharmacology. 2017 Jul;53:151–7. |
| 27 | Jeong KS, Park H, Ha E, Hong Y-C, Ha M, Park H, et al. Performance IQ in children is associated with blood cadmium concentration in early pregnancy. Journal of Trace Elements in Medicine and Biology. 2015 Apr;30:107–11. |
| 28 | Macias-Barragan J, Huerta-Olvera SG, Hernandez-Cañaveral I, Pereira-Suarez AL, Montoya-Buelna M. Cadmium and α-lipoic acid activate similar de novo synthesis and recycling pathways for glutathione balance. Environmental Toxicology and Pharmacology. 2017 Jun;52:38–46 |
| 29 | Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. Journal of Cellular Biochemistry. 2018 Jan;119(1):157–84. |
| 30 | Shome S, Talukdar AD, Choudhury MD, Bhattacharya MK, Upadhyaya H. Curcumin as potential therapeutic natural product: a nanobiotechnological perspective. Journal of Pharmacy and Pharmacology. 2016 Dec;68(12):1481–500. |
| 31 | Eberhart J, Lovely C, Rampersad M, Fernandes Y. Gene-environment interactions in development and disease. Wiley Interdiscip Rev Dev Biol [Internet]. 2017 Jan;6(1). |
| 32 | Edwards M. Fetal Death and Reduced Birth Rates Associated with Exposure to Lead-Contaminated Drinking Water. Environmental Science & Technology. 2014 Jan 7;48(1):739–46. |
| 33 | Liu Z, Yu Y, Li X, Wu A, Mu M, Li N, et al. Maternal lead exposure and risk of congenital heart defects occurrence in offspring. Reproductive Toxicology. 2015 Jan;51:1–6. |
| 34 | Ou Y, Bloom MS, Nie Z, Han F, Mai J, Chen J, et al. Associations between toxic and essential trace elements in maternal blood and fetal congenital heart defects. Environment International. 2017 Sep;106:127–34. |
| 35 | Rabito FA, Kocak M, Werthmann DW, Tylavsky FA, Palmer CD, Parsons PJ. Changes in low levels of lead over the course of pregnancy and the association with birth outcomes. Reproductive Toxicology. 2014 Dec;50:138–44. |
| 36 | Wang H, Li J, Hao J-H, Chen Y-H, Liu L, Yu Z, et al. High serum lead concentration in the first trimester is associated with an elevated risk of small-for-gestational-age infants. Toxicology and Applied Pharmacology. 2017 Oct;332:75–80. |
| 37 | Jin X, Tian X, Liu Z, Hu H, Li X, Deng Y, et al. Maternal exposure to arsenic and cadmium and the risk of congenital heart defects in offspring. Reproductive Toxicology. 2016 Jan;59:109–16. |
| 38 | Minatel BC, Sage AP, Anderson C, Hubaux R, Marshall EA, Lam WL, et al. Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environment International. 2018 Mar;112:183–97. |
| 39 | Saint-Jacques N, Brown P, Nauta L, Boxall J, Parker L, Dummer TJB. Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environment International. 2018 Jan;110:95–104. |
| 40 | Hosp C, Hamm H. Safety of available and emerging drug therapies for hyperhidrosis. Expert Opinion on Drug Safety. 2017 Sep 2;16(9):1039–49. |
| 41 | Jones K, Linhart C, Hawkins C, Exley C. Urinary Excretion of Aluminium and Silicon in Secondary Progressive Multiple Sclerosis. EBioMedicine. 2017 Dec;26:60–7. |
| 42 | Mirza A, King A, Troakes C, Exley C. Aluminium in brain tissue in familial Alzheimer’s disease. Journal of Trace Elements in Medicine and Biology. 2017 Mar;40:30–6. |
| 43 | Mold M, Umar D, King A, Exley C. Aluminium in brain tissue in autism. Journal of Trace Elements in Medicine and Biology. 2018 Mar;46:76–82. |
| 44 | Flora SJS, Shrivastava R, Mittal M. Chemistry and pharmacological properties of some natural and synthetic antioxidants for heavy metal toxicity. Curr Med Chem. 2013;20(36):4540–74. |
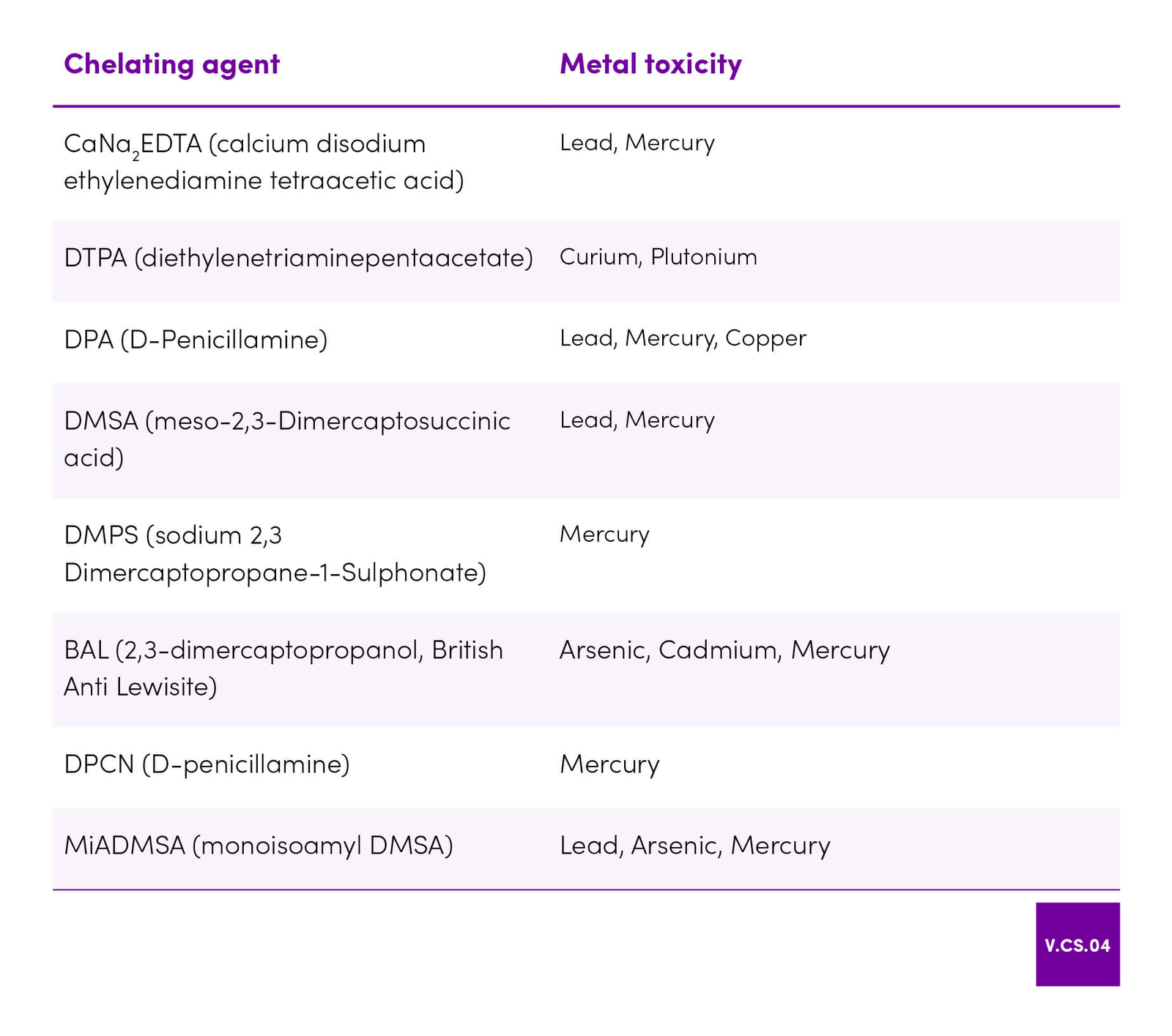
| 45 | Aaseth J, Skaug MA, Cao Y, Andersen O. Chelation in metal intoxication—Principles and paradigms. Journal of Trace Elements in Medicine and Biology. 2015 Jul;31:260–6. |
| 46 | Li B, Jin D, Yu S, Etareri Evivie S, Muhammad Z, Huo G, et al. In Vitro and In Vivo Evaluation of Lactobacillus delbrueckii subsp. bulgaricus KLDS1.0207 for the Alleviative Effect on Lead Toxicity. Nutrients [Internet]. 2017 Aug 8;9(8). |
| 47 | Yadav A, Mathur R, Samim M, Lomash V, Kushwaha P, Pathak U, et al. Nanoencapsulation of DMSA monoester for better therapeutic efficacy of the chelating agent against arsenic toxicity. Nanomedicine. 2014 Mar;9(4):465–81. |
| 48 | Flora SJS, Pachauri V. Chelation in Metal Intoxication. International Journal of Environmental Research and Public Health. 2010 Jun 28;7(7):2745–88. |
| 49 | Rafati-Rahimzadeh M, Rafati-Rahimzadeh M, Kazemi S, Moghadamnia AA. Current approaches of the management of mercury poisoning: need of the hour. Daru. 2014 Jun 2;22(1):46. |
| 50 | Aranda N, Valls RM, Romeu M, Sánchez-Martos V, Albaladejo R, Fernández-Castillejo S, et al. Consumption of seafood and its estimated heavy metals are associated with lipid profile and oxidative lipid damage on healthy adults from a Spanish Mediterranean area: A cross-sectional study. Environmental Research. 2017 Jul;156:644–51. |
| 51 | FSANZ. Mercury in fish [Internet]. 2011 [cited 2018 Aug 9]. Available from: http://www.foodstandards.gov.au/consumer/chemicals/mercury/Pages/default.aspx |
| 52 | Food Authority, NSW Government. Mercury and fish [Internet]. 2018 [cited 2018 Aug 9]. Available from: /foodsafetyandyou/life-events-and-food/pregnancy/mercury-and-fish |
| 53 | Rendón-Ramírez A-L, Maldonado-Vega M, Quintanar-Escorza M-A, Hernández G, Arévalo-Rivas B-I, Zentella-Dehesa A, et al. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environmental Toxicology and Pharmacology. 2014 Jan;37(1):45–54. |
| 54 | Colovic MB, Vasic VM, Djuric DM, Krstic DZ. Sulphur-containing Amino Acids: Protective Role Against Free Radicals and Heavy Metals. Curr Med Chem. 2018 Jan 30;25(3):324–35. |
| 55 | Liu K, Zheng J, Chen F. Effects of washing, soaking and domestic cooking on cadmium, arsenic and lead bioaccessibilities in rice: Effects of cooking on Cd, As and Pb bioaccessibilities in rice. Journal of the Science of Food and Agriculture. 2018 Aug;98(10):3829–35. |
| 56 | Pastorelli AA, Angeletti R, Binato G, Mariani MB, Cibin V, Morelli S, et al. Exposure to cadmium through Italian rice ( Oryza sativa L.): Consumption and implications for human health. Journal of Food Composition and Analysis. 2018 Jun;69:115–21. |
| 57 | Fransisca Y, Small DM, Morrison PD, Spencer MJS, Ball AS, Jones OAH. Assessment of arsenic in Australian grown and imported rice varieties on sale in Australia and potential links with irrigation practises and soil geochemistry. Chemosphere. 2015 Nov;138:1008–13. |
| 58 | Xu L, Zhang W, Liu X, Zhang C, Wang P, Zhao X. Circulatory Levels of Toxic Metals (Aluminum, Cadmium, Mercury, Lead) in Patients with Alzheimer’s Disease: A Quantitative Meta-Analysis and Systematic Review. Journal of Alzheimer’s Disease. 2018 Feb 6;62(1):361–72. |
| 59 | Davenward S, Bentham P, Wright J, Crome P, Job D, Polwart A, et al. Silicon-Rich Mineral Water as a Non-Invasive Test of the ‘Aluminum Hypothesis’ in Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2012 Dec 17;33(2):423–30. |
| 60 | Rebelo FM, Caldas ED. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environmental Research. 2016 Nov;151:671–88. |
| 61 | Park Y, Lee A, Choi K, Kim H-J, Lee JJ, Choi G, et al. Exposure to lead and mercury through breastfeeding during the first month of life: A CHECK cohort study. Science of The Total Environment. 2018 Jan;612:876–83. |
| 62 | Letinić G, Sarić M, Piasek M, Jurasović J, Varnai V, Grgec S, et al. Use of human milk in the assessment of toxic metal exposure and essential element status in breastfeeding women and their infants in coastal Croatia. J Trace Elem Med Biol. 2016 Dec;38:117–25. |
| 63 | Chao H-H, Guo C-H, Huang C-B, Chen P-C, Li H-C, Hsiung D-Y, et al. Arsenic, Cadmium, Lead, and Aluminium Concentrations in Human Milk at Early Stages of Lactation. Pediatrics & Neonatology. 2014 Apr;55(2):127–34. |
| 64 | Cherkani-Hassani A, Ghanname I, Mouane N. Assessment of cadmium levels in human breast milk and the affecting factors: A systematic review, 1971–2014. Critical Reviews in Food Science and Nutrition. 2017 Jul 24;57(11):2377–91. |
| 65 | Al-Saleh I, Elkhatib R, Al-Rouqi R, Abduljabbar M, Eltabache C, Al-Rajudi T, et al. Alterations in biochemical markers due to mercury (Hg) exposure and its influence on infant’s neurodevelopment. International Journal of Hygiene and Environmental Health. 2016 Nov;219(8):898–914. |
| 66 | adán-Piedra C, Vélez D, Devesa V. In vitro evaluation of dietary compounds to reduce mercury bioavailability. Food Chemistry. 2018 May;248:353–9. |
| 67 | Jadán Piedra C, Sánchez V, Vélez D, Devesa V. Reduction of mercury bioaccessibility using dietary strategies. LWT - Food Science and Technology. 2016 Sep;71:10–6. |
| 68 | Silva de Paula E, Carneiro MFH, Grotto D, Hernandes LC, Antunes LMG, Barbosa F. Protective effects of niacin against methylmercury-induced genotoxicity and alterations in antioxidant status in rats. Journal of Toxicology and Environmental Health, Part A. 2016 Feb 16;79(4):174–83. |
| 69 | Li X, Jiang X, Sun J, Zhu C, Li X, Tian L, et al. Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity: Mechanisms of dietary flavonoids against cadmium. Annals of the New York Academy of Sciences. 2017 Jun;1398(1):5–19. |
| 70 | Brzóska MM, Rogalska J, Galazyn-Sidorczuk M, Jurczuk M, Roszczenko A, Tomczyk M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chemico-Biological Interactions. 2015 Mar;229:132–46. |
| 71 | Gong P, Chen F, Wang L, Wang J, Jin S, Ma Y. Protective effects of blueberries (Vaccinium corymbosum L.) extract against cadmium-induced hepatotoxicity in mice. Environmental Toxicology and Pharmacology. 2014 May;37(3):1015–27. |
| 72 | Amamou F, Nemmiche S, Meziane R kaouthar, Didi A, Yazit SM, Chabane-Sari D. Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food and Chemical Toxicology. 2015 Apr;78:177–84. |
| 73 | Chaudhary S, Iram S, Raisuddin S, Parvez S. Manganese pre-treatment attenuates cadmium induced hepatotoxicity in Swiss albino mice. Journal of Trace Elements in Medicine and Biology. 2015 Jan;29:284–8. |
| 74 | Abdelaziz I, Elhabiby M, Ashour A. Toxicity of cadmium and protective effect of bee honey, vitamins C and B complex. Human & Experimental Toxicology. 2013 Apr;32(4):362–70. |
| 75 | Hernández-Plata E, Quiroz-Compeán F, Ramírez-Garcia G, Barrientos EY, Rodríguez-Morales NM, Flores A, et al. Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment. Toxicology Letters. 2015 Mar;233(2):78–83. |
| 76 | Chandrasekaran VRM, Hsu D-Z, Liu M-Y. Beneficial Effect of Sesame Oil on Heavy Metal Toxicity. Journal of Parenteral and Enteral Nutrition. 2014 Feb;38(2):179–85. |
| 77 | El-Khishin IA, El-fakharany YMM, Abdel Hamid OI. Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicology Reports. 2015;2:824–32. |
| 78 | Krempaská K, Vaško L, Vašková J. Humic Acids as Therapeutic Compounds in Lead Intoxication. Curr Clin Pharmacol. 2016;11(3):159–67. |
| 79 | Zhang J, Zhou X, Wu W, Wang J, Xie H, Wu Z. Regeneration of glutathione by α-lipoic acid via Nrf2/ARE signaling pathway alleviates cadmium-induced HepG2 cell toxicity. Environmental Toxicology and Pharmacology. 2017 Apr;51:30–7. |
| 80 | Lee M, Cho S, Roh K, Chae J, Park J-H, Park J, et al. Glutathione alleviated peripheral neuropathy in oxaliplatin-treated mice by removing aluminum from dorsal root ganglia. Am J Transl Res. 2017 Mar 15;9(3):926–39. |
| 81 | Wang X, Fan X, Yuan S, Jiao W, Liu B, Cao J, et al. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food & Function. 2017;8(8):2924–34 |
| 82 | Sharma DR, Wani WY, Sunkaria A, Kandimalla RJ, Sharma RK, Verma D, et al. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience. 2016 Jun;324:163–76. |
| 83 | Kinoshita H, Sohma Y, Ohtake F, Ishida M, Kawai Y, Kitazawa H, et al. Biosorption of heavy metals by lactic acid bacteria and identification of mercury binding protein. Research in Microbiology. 2013 Sep;164(7):701–9. |
| 84 | Zhai Q, Yin R, Yu L, Wang G, Tian F, Yu R, et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015 Aug;54:23–30. |
| 85 | Yi Y-J, Lim J-M, Gu S, Lee W-K, Oh E, Lee S-M, et al. Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb(II) toxicity. Journal of Microbiology. 2017 Apr;55(4):296–303. |
| 86 | Ojekunle O, Banwo K, Sanni AI. In vitro and in vivo evaluation of Weissella cibaria and Lactobacillus plantarum for their protective effect against cadmium and lead toxicities. Letters in Applied Microbiology. 2017;64(5):379–85. |
| 87 | Yu L, Zhai Q, Tian F, Liu X, Wang G, Zhao J, et al. Potential of Lactobacillus plantarum CCFM639 in Protecting against Aluminum Toxicity Mediated by Intestinal Barrier Function and Oxidative Stress. Nutrients [Internet]. 2016 Dec 2;8(12). |
| 88 | Tinkov AA, Gritsenko VA, Skalnaya MG, Cherkasov SV, Aaseth J, Skalny AV. Gut as a target for cadmium toxicity. Environmental Pollution. 2018 Apr;235:429–34. |
| 89 | Rennolds J, Malireddy S, Hassan F, Tridandapani S, Parinandi N, Boyaka PN, et al. Curcumin regulates airway epithelial cell cytokine responses to the pollutant cadmium. Biochemical and Biophysical Research Communications. 2012 Jan;417(1):256–61. |
| 90 | Kukongviriyapan U, Pannangpetch P, Kukongviriyapan V, Donpunha W, Sompamit K, Surawattanawan P. Curcumin Protects against Cadmium-Induced Vascular Dysfunction, Hypertension an |
| 91 | Mohajeri M, Rezaee M, Sahebkar A. Cadmium-induced toxicity is rescued by curcumin: A review: Cadmium-induced toxicity. BioFactors. 2017 Sep 10;43(5):645–61. |
| 92 | Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G. Lead Toxicity, Antioxidant Defense and Environment. In: de Voogt P, editor. Reviews of Environmental Contamination and Toxicology Volume 238 [Internet]. Cham: Springer International Publishing; 2016. p. 45–67 |
| 93 | Pal M, Sachdeva M, Gupta N, Mishra P, Yadav M, Tiwari A. Lead Exposure in Different Organs of Mammals and Prevention by Curcumin–Nanocurcumin: a Review. Biological Trace Element Research. 2015 Dec;168(2):380–91. |
| 94 | Liu W, Xu Z, Li H, Guo M, Yang T, Feng S, et al. Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation. Human & Experimental Toxicology. 2017 Sep;36(9):949–66. |
| 95 | Sankar P, Telang AG, Kalaivanan R, Karunakaran V, Suresh S, Kesavan M. Oral nanoparticulate curcumin combating arsenic-induced oxidative damage in kidney and brain of rats. Toxicology and Industrial Health. 2016 Mar;32(3):410–21. |
| 96 | Winiarska-Mieczan A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure – A rat model study. Regulatory Toxicology and Pharmacology. 2015 Nov;73(2):521–9. |
| 97 | Khalaf AA, Moselhy WA, Abdel-Hamed MI. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. NeuroToxicology. 2012 Jun;33(3):280–9. |
| 98 | Chen L, Mo H, Zhao L, Gao W, Wang S, Cromie MM, et al. Therapeutic properties of green tea against environmental insults. J Nutr Biochem. 2017 Feb;40:1–13. |
![]() lipid profile and elevated levels of oxidised LDL (50).
lipid profile and elevated levels of oxidised LDL (50).