
Children and adolescents with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are generally considered to have a lower risk of hospitalisation and lower mortality rates than adults (1). However, since late 2020, concerns regarding the long-term health effects of SARS-CoV-2 infection in children have emerged (2). Evidence from case studies, patient support groups and clinicians highlights that children with asymptomatic and symptomatic coronavirus disease-19 (COVID-19) are experiencing long-term effects weeks to months after the initial infection (3,4,5).
Two long-term consequences of SARS-CoV-2 infection in children raise concern: multisystem inflammatory syndrome in children (MIS-C) and post-acute sequelae of COVID-19 (PASC), also known as long COVID (4). MIS-C is a rare paediatric hyperinflammatory disease with Kawasaki disease and Toxic Shock Syndrome features that typically occurs 2–6 weeks after SARS-CoV-2 infection (6,7,8). It develops in less than 0.1% of children with COVID-19 and requires intensive care support in 68% (9). In children who acquire MIS-C, organs and tissues including the heart, blood vessels, kidneys, lungs, nervous system, digestive system, skin, and eyes, can become severely inflamed (10).
Children are thought to be less vulnerable than adults to long COVID. However, the emergence of the Omicron variant and its subvariants, which are far more infectious than previous forms of SARS-CoV-2, has led to a higher proportion of children becoming infected. Epidemiologists estimate that if even one per cent of infected children develop long-term symptoms, this could mean millions of children globally will need ongoing healthcare (11,12).
Clinicians worldwide have reported increasing numbers of children and adolescents diagnosed with post-COVID conditions in the last six months. For example, the latest data from the UK's Office of National Statistics found that the number of children under 16 with self-reported Long COVID of any duration increased from 77,000 in October 2021 to 149,000 in March 2022 (13). However, it is unclear if these cases are due to long-term biological effects of SAR-CoV-2 infection, the psychological effects of lockdowns, or other causes, due to the lack of studies on paediatric long COVID.
The lack of a paediatric definition of long COVID has hindered research and diagnosis, appropriate clinical management and treatment of children suffering long-term symptoms after SAR-CoV-2 infection. However, the first standardised definition of long COVID in children and adolescents has recently been developed and complements the clinical case definition in adults proposed by the World Health Organisation (Table 1) (14). Adopting this definition would allow researchers to reliably compare and evaluate studies on prevalence, disease course and outcome of long COVID.
Table 1. Research definition of post-COVID-19 condition (Long COVID) among children and young people (14)
|
|
|
|
Definition |
Post-COVID-19 condition occurs in young people with a history of confirmed SARS-CoV-2 infection with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time |
|
Key points |
A condition in which a child or young person has symptoms (at least one of which is a physical symptom) that:
|
The evidence
Prevalence
Nearly 30 studies, including cross-sectional studies, prospective cohort studies, case series and retrospective cohort studies, have investigated persistent symptoms of SARS-CoV-2 in children and adolescents. However, the prevalence of long COVID in children and adolescents reported in these studies varies widely, ranging from 4% to 66% (4). In the only Australian study published to date following 171 children in a dedicated post-COVID-19 clinic between 21 March 2020 and 17 March 2021, 8% reported post-COVID symptoms up to eight weeks (15).
The variability arises from a lack of paediatric definition of long COVID and heterogeneity in the studies, including differences in the sample size, the median age of the included population, duration, and follow-up. Furthermore, most studies are uncontrolled; they lack a SARS-CoV-2 negative control group and, therefore, may overestimate the real burden of long COVID.
The few controlled studies to date have produced conflicting results, with some studies reporting no difference in the prevalence of long-lasting symptoms between children who tested positive for SARS-CoV-2 compared to those who tested negative (16,17).
A summary of controlled studies is provided below:
- A Latvian study comparing 236 paediatric COVID-19 patients to 142 comparison controls found that after a median follow-up of 73.5 days, 70% of COVID-19 patients had at least one persisting symptom, and 53% had two or more. Compared to controls, long-term persistent symptoms such as fever, fatigue, rhinorrhoea, loss of taste and/or smell, headaches, cognitive sequelae, and nocturnal sweating were more common in COVID-19 patients (18).
- In the LongCOVIDKidsDK study, 61.9% of adolescents with a positive SARS-CoV-2 test experienced at least one symptom lasting two months compared to 57% in the control group (19).
- A Danish cohort study (n=37,522) found that 0.8% of SARS-CoV-2 positive children aged 6-17 years reported symptoms lasting >4 weeks compared to a control group. Depending on age, symptoms resolved in 54-75% of children within one to five months (17).
- In England and Wales, the VirusWatch study showed that symptoms lasting for more than four weeks were reported by 4.6% of children with a history of SARS-CoV-2 compared to 1.7% of controls (20).
- A UK study using the UK ZOE COVID Symptom Study app reported that 4.4% of children still had symptoms four weeks after COVID-19 onset, which decreased to 1.8% at eight or more weeks. Less than 1% of the control group was symptomatic for more than 28 days (21).
- In the Children & Young People with Long Covid (CLoCk) Study (n=6,804), a national cohort study of adolescents aged 11-17 years, both children with a positive SARS-CoV-2 test (66.5%) and those without (53.3%) reported symptoms at three months after SARS-CoV-2 testing, although those who tested positive were nearly twice as likely to report three or more symptoms (30.3% vs. 16.2%) (22).
- In a Swiss longitudinal cohort study, 9% of SARS-CoV-2 positive children compared to 10% of uninfected children reported at least one symptom lasting more than four weeks. For symptoms lasting longer than 12 weeks, 2% of SARS-CoV-2 positive children reported one or more symptoms compared to 4% of uninfected children (16).
These findings highlight the importance of including a control group to distinguish symptoms arising from SARS-CoV-2 infection from other potential causes, such as other paediatric viruses or illnesses and pandemic-associated symptoms.
Clinical presentation
The clinical presentation of long COVID in children and adolescents is also highly variable in terms of symptoms, intensity, and duration. Symptoms can occur in isolation or combination and remain constant, transient, or have an intermittent-relapse pattern. Furthermore, symptoms may not appear until weeks after the initial infection (1,3,4).
The prevalence of clinical manifestations in children with long COVID varies widely in the literature (Table 2). Some studies suggest that long COVID symptoms seem to be more common in children with symptomatic or severe acute SARS-CoV-2 infection, although emerging evidence indicates that asymptomatic children may also develop long COVID (1,3,4). As with adults, fatigue is the most frequently reported symptom in children (up to 87%) and has the potential to severely impact daily functioning, quality of life and school attendance (4).
Table 2. Prevalence of clinical manifestations in children with long COVID (4)
|
System |
Sign or symptom |
|
Neurological |
Brain fog Concentration difficulties (2-81%) Sleep disturbance (2-63%) Dizziness (3-20%) Irritability and mood changes (5-24%) Headache (3-80%) Memory loss Smell disorder (12-70%) Taste disorder (20-70%) Nocturnal sweating |
|
Respiratory |
Cough (1-30%) Dyspnoea (40-50%) Nasal congestion or rhinorrhoea (1-12%) Sore throat (4-70%) |
|
Cardiovascular |
Chest tightness or pan (1-31%) Palpitations (4-18%) |
|
Dermatological |
Skin rashes (2-52%) |
|
Gastrointestinal |
Stomach-ache (5-70%) Abdominal pain (1-76%) Diarrhoea (2-24%) Vomiting (2-24%) |
|
General |
Fatigue (3-87%) Persistent fever (2-40%) Loss of appetite or weight (2-50%) |
|
Muscular |
Myalgia or arthralgia (1-61%) |
A recent systematic review and meta-analysis of 22 studies identified 101 long COVID symptoms in children and adolescents involving the cardiovascular, respiratory, gastrointestinal, musculoskeletal, skin and nervous systems and general somatic symptoms (3). Data were sufficient to examine 14 of the most common symptoms in controlled studies; and meta-analyses found significantly higher pooled proportions in cases with confirmed SARS-CoV-2 for loss of smell (8%), headaches (5%), cognitive difficulties (3%), and sore throat and eyes (2% each). However, the frequency of abdominal pain, cough, myalgia, insomnia, diarrhoea, fever, and dizziness was similar in SARS-CoV-2 positive cases and controls.
Several studies have demonstrated the transient nature of long COVID symptoms in children. For example, in the Long COVID Kids Rapid Survey 2 (n=510), 25.3% of children experienced constant COVID-19 infection symptoms, 49.4% had had periods of apparent recovery followed by a return of symptoms, and 19.0% had a prolonged period of wellness followed by the return of symptoms (23). Of concern, the researchers found that most children had worse activity levels than before infection, as reflected by the fact that, at the time of the survey, 21.2% were unable to perform activity, and 30.2% enjoyed the occasional activity but usually had an increase in symptoms afterwards.
A noteworthy finding of the CloCk study is that more than 50% of children who did not have COVID-19 experienced headaches, fatigue, sleep disturbance and concentration difficulties during the pandemic (22). Furthermore, no difference was reported between the two groups in impairment of activities, mental health, or overall wellbeing. This study highlights the importance of including appropriate control groups and the complexity of distinguishing long COVID symptoms from pandemic-associated symptoms arising from lockdowns, school closures and social restrictions.
Limitations
Results of the observational studies and meta-analyses need to be treated with caution given the following limitations:
- The lack of a paediatric definition of long COVID means studies have used variable inclusion criteria and follow-up times.
- Studies have substantial heterogeneity, including the age of participants, pre-existing medical conditions, disease severity, standard care treatment strategies, laboratory confirmed COVID, time points of assessment, number and duration of symptoms and follow up.
- Most studies included self-, or parent-reported symptoms from questionnaires without clinical assessment and objective parameters, e.g., lung function.
- Due to the lack of a control group in the majority of studies, therefore it is impossible to distinguish symptoms of long COVID from symptoms attributable to the pandemic, e.g. school closures.
- Selection bias – as those with persisting symptoms might be more likely to respond to surveys, this can lead to a substantial overestimation of the prevalence of long COVID.
- There is the possibility that the uninfected control group may be contaminated by asymptomatic children or those with mild symptoms that may not have been tested.
- Almost all studies (95%) were from high-income countries, limiting generalisability for low and middle-income countries.
- All studies have the limitations inherent in all observational studies, including bias due to residual and unmeasured confounding.
Biological mechanisms
Children have several protective factors leading to a milder severity and duration of COVID-19, including fewer angiotensin converting enzyme-2 (ACE2) receptors, fewer co-morbidities and age-related endothelial damage, strong innate immune response, and active thymic function, which leads to the increased presence and decreased depletion of T cells which recognise viral proteins (24,25). Other protective factors include environmental or non-inheritable factors, including past infections, vaccines, nutrition, and the gut microbiome (26). These protective factors during acute infection may also be protective against long COVID in children and adolescents; however, further research is required.
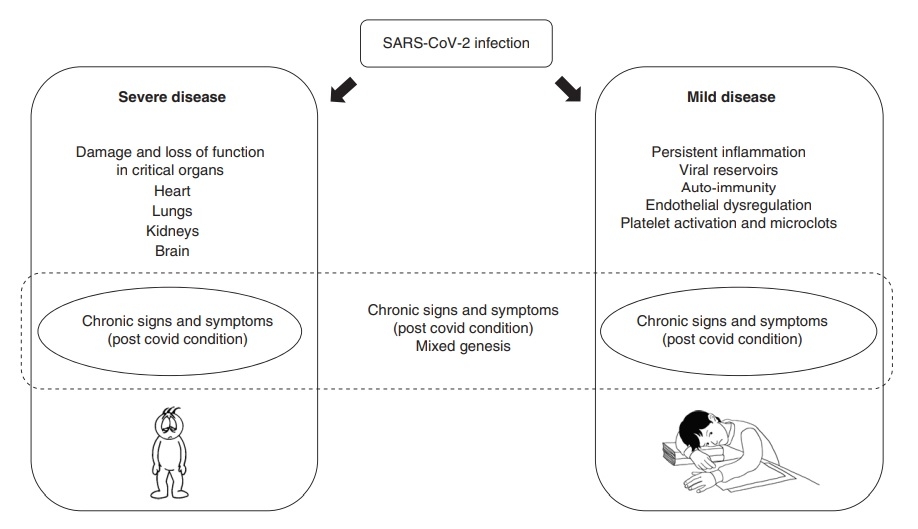
The underlying mechanisms causing the various clinical spectrum of long COVID are still unknown but may be divided into two main groups: 1) related to organ damage during the acute illness and 2) other, less well-characterised mechanisms, which may include chronic tissue damage, immunological and inflammatory mechanisms, as well viral persistence, autoimmunity, chronic inflammation, changes in the autonomic nervous system and coagulation issues (Figure 1) (27,28,29,30).
Gut dysbiosis and loss of intestinal permeability have also been identified in patients with long COVID (31,32). For example, in a recent study of children, a prolonged presence of SARS-CoV-2 in the gastrointestinal tract led to the release of zonulin, a biomarker of intestinal permeability, with subsequent trafficking of SARS-CoV-2 antigens into the bloodstream, leading to hyperinflammation (33).
Many mechanisms and risk factors appear to be similar in adults and children, but targeted studies are needed to understand the pathophysiology of long COVID in children. Risk factors in both adult and paediatric populations include older age, female gender, severe COVID-19, overweight/obesity, and other long-term co-morbidities (3,4). In children, allergic diseases are also associated with a higher risk of long COVID, leading researchers to propose that mast cell activation syndrome and a T helper 2 (Th2) dominant immunological response could be involved in the pathogenesis of long COVID (34,35,36).
Figure 1. Possible mechanisms behind long COVID (2) CC BY 4.0
Conclusion
There is increasing evidence that children can be affected by long-term sequelae after COVID- 19 infection; however, the paucity of studies and the limitations of studies published to date mean the true prevalence of this syndrome in children and adolescents remains uncertain. Regardless, the studies to date highlight an emerging public health crisis and the need for systematic multidisciplinary assessment of children and adolescents with persistent symptoms after a SARS-CoV-2 acute infection and those affected by pandemic-associated symptoms.
There is an urgent need for longitudinal cohort studies with rigorous control groups to understand the true prevalence and clinical presentation of long COVID in children and adolescents. These studies need to include regular testing for SARS-CoV-2 to confirm infection, the capture of symptoms, follow-up times that are both consistent and of sufficient duration to account for intermittent symptoms and recording of relevant information such as pre-existing medical conditions, vaccination status and SARS-CoV-2 variant, if possible. Furthermore, research into the aetiology and pathophysiology of long COVID and interventional treatment trials, including children and adults, is needed to inform treatment strategies and improve long term COVID patient outcomes.






