A recent study indicates that following a diet rich in prebiotic and fermented foods, termed a psychobiotic diet, can reduce stress and improve sleep (1).
Over the past decade, a growing body of research has shown that diet can significantly impact neurological function, cognition and mental health via processes involving the communication pathways between the gut microbiome and the brain (the microbiota–gut–brain axis) (2). These findings led to new research into microbiome-targeted therapies termed psychobiotics. These therapies have a bacterially mediated impact on the brain and behaviour and include prebiotics, probiotics, postbiotics ![]() and dietary interventions (3,4,5).
and dietary interventions (3,4,5).
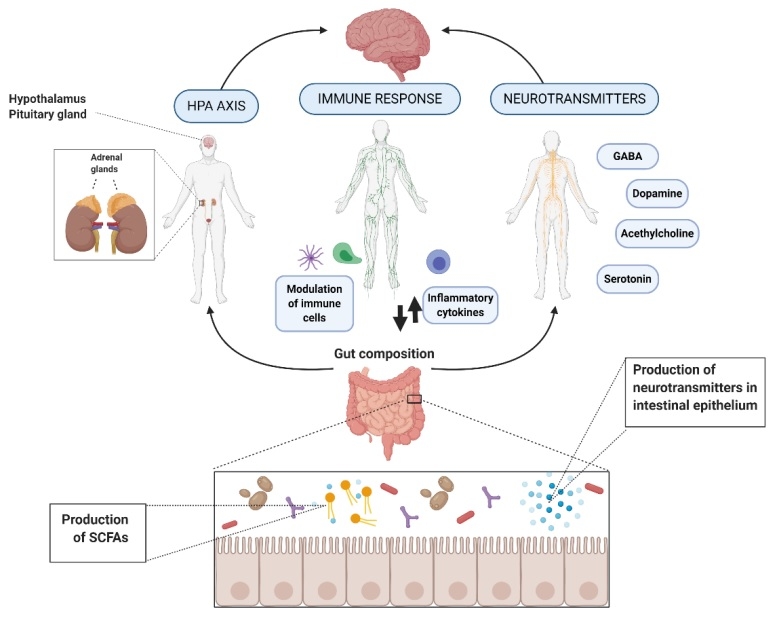
Microorganisms can influence central nervous system (CNS) processes bidirectionally via the vagus nerve (6) and through modulation of the immune system (7), the hypothalamic-pituitary-adrenal (HPA) axis (8), and tryptophan metabolism, along with their ability to synthesise several neurotransmitters (9,10) and produce metabolites, such as short-chain fatty acids (SCFAs). Experimental studies suggest that these microbiota-mediated mechanisms could underlie the diet–brain connection (11). Human clinical studies have demonstrated the efficacy of microbiota-targeted dietary approaches, such as the Mediterranean diet, for improving mental health and cognition (12,13,14). However, the majority of these studies did not examine microbiota compositional changes, and further research is required to assess the association between diet, gut microbiome and mental health (15).
Figure 1. Action of mechanisms by which the gut microbiota exert the potential psychobiotic effect (11) CC BY 4.0
In a recent four-week randomised controlled trial (RCT), 45 healthy adults aged 18 to 59 years were randomised into two groups: an intervention group that followed a psychobiotic diet and a control group that followed a diet based on the healthy eating food pyramid.
All participants consumed a low-fibre diet, with an average intake of less than 15 g/day, which is well below the recommended daily intake of 30/day for men and 25 g/day for women (16).
The psychobiotic diet included 6-8 servings daily of fruits and vegetables high in prebiotic fibres (such as onions, leeks, cabbage, apples, bananas and oats), 5-8 servings of grains per day, 3-4 servings of legumes per week and 2-3 servings of fermented foods daily (such as sauerkraut, kefir and kombucha).
Stress, overall health, sleep and diet were assessed using validated questionnaires. Results show that a psychobiotic diet significantly reduces stress. After one month, 32% of participants on the psychobiotic diet had reduced perceived stress scores, compared to only 17% of participants on the control diet. The reduction in perceived stress was dose-dependent, meaning that higher adherence to the psychobiotic diet resulted in greater decreases in stress scores. The quality of sleep improved in both groups; however, only participants on the psychobiotic diet had a statistically significant improvement in subjective sleep quality.
To assess the potential mechanisms underlying the microbiota-gut-brain communication, faecal, urinary and blood specimens were provided before and during the study. The faecal samples were analysed to determine microbiota composition and function. In addition, urinary and blood samples were analysed for key metabolites resulting from human-gut microbiota co-metabolism of dietary essential amino acids tryptophan, tyrosine, phenylalanine, and branched-chain amino acids.
Morning cortisol levels and inflammatory markers were not significantly affected by the psychobiotic dietary intervention, suggesting short-term dietary intervention did not alter perceived stress via effects on the immune system or HPA axis. In addition, microbiota composition and function did not significantly vary in the psychobiotic group, which may have been due to the short study duration.
Significant changes in the level of 40 specific faecal lipids were observed. Recent experimental studies have reported associations between changes in lipid metabolites, and depressive-like behaviour, suggesting that the microbiota might influence mood by regulating lipid metabolism (17,18).
In the psychobiotic dietary group, there was a significant decrease in urinary metabolites of quinolinic acid, L-tryptophan, and L-phenylalanine. Tryptophan is an essential amino acid required to produce melatonin, which helps regulate the sleep-wake cycle, and serotonin, a neurotransmitter that helps regulate appetite, mood, sleep, and pain. This amino acid can be metabolised in two pathways relevant to depression and other neuropsychiatric disorders: the serotonin and kynurenine pathways (19). In the current study, the psychobiotic diet reduced the kynurenine pathway metabolite, quinolinic acid. This metabolite is an N-methyl-d-aspartate (NMDA) receptor agonist and can exert neurotoxic effects, which can disrupt neurotransmission and cause depressive symptoms (20,21).
The study had several limitations, including the limited sample size, short study duration, and the use of food frequency questionnaires which are susceptible to measurement error and bias in estimating food intake. Nevertheless, the results suggest that dietary interventions targeting the gut microbiota can reduce stress and improve sleep, potentially through the effects on tryptophan metabolism and lipid metabolites. More extensive and longer-duration studies are warranted to confirm the stress-alleviating effect of the psychobiotic diet and identify the underlying mechanistic pathways.