A new study suggests the body produces higher than normal amounts of autoantibodies ![]() months after contracting even mild severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection, adding to the research relating COVID-19 disease to autoimmune processes (1).
months after contracting even mild severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection, adding to the research relating COVID-19 disease to autoimmune processes (1).
Around 30-70% of COVID-19 patients suffer from post-acute sequelae of COVID-19 (PASC) or long COVID (2). Many of the signs and symptoms that persist for weeks to months after initial SARS-CoV-2 infection, including fatigue, myalgia, cognitive dysfunction (“brain fog”), breathlessness and reduced exercise capacity, have overlapping similarities with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (3,4).
Furthermore, long COVID symptoms are reported to have an unpredictable flare and remission pattern and share immunological and clinical features with other autoimmune diseases, such as systemic autoimmune rheumatic diseases (SARDs), including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (5).
The underlying mechanisms for long COVID are still undefined, but a sustained inflammatory response caused by the persistence of SARS-CoV-2 in tissue and organs, or autoimmune processes are two of the most considered hypotheses (6).
The current prospective study compared blood samples from 177 individuals with confirmed evidence of prior SARS‑CoV‑2 infection to pre-pandemic samples collected from 53 healthy individuals. In addition, demographic data and COVID-19 related symptoms experienced up to 6 months prior to sample collection were collected using an electronic survey.
Autoantibody reactivity to 91 autoantigens previously linked to a range of classic autoimmune diseases was assessed, and statistical analysis was undertaken to examine the associations between autoantibody reactivity of each antigen and self-reported symptom burden.
The results showed that all 177 individuals had persistent autoantibodies up to six months after acute SARS-CoV-2 infection; however, sex-specific variations were detected. For example, the overall autoantibody response was more prominent in women than men following asymptomatic infection. By contrast, among previously infected individuals who experienced at least a mild burden of symptoms, the extent of autoantibody response was greater in men.
Some of the autoantibodies identified in the study have been linked to autoimmune diseases that typically affect women more often than men, such as SLE and RA. In this study, however, men had a higher number of elevated autoantibodies than women, which may be related to sex differences in the immune response during the acute stage of SARS-CoV-2 infection (7).
Previous studies have reported that more than 50% of hospitalised COVID-19 patients exhibited increases in autoantibody reactivities as compared to uninfected individuals and showed a high prevalence of autoantibodies against immunomodulatory proteins (such as type 1 interferons), which can impair the innate antiviral response and is associated with COVID-19 disease severity (8). However, the current study is the first to show that autoantibodies persist up to 6 months after active SARS-CoV-2 infection.
A recent study found that the presence of specific autoantibodies at the time of COVID-19 diagnosis was a risk factor for experiencing long COVID symptoms 2 to 3 months after initial infection. These included autoantibodies targeting interferon alpha-2 (anti-IFN-α2) and anti-nuclear autoantibodies (ANAs) commonly associated with SLE. Furthermore, specific autoantibodies were associated with different long COVID symptoms. For example, anti-IFN-α2 at the time of diagnosis forecasted respiratory symptoms of long COVID (9). In another study, the frequency of neurocognitive symptoms 12 months after SARS-CoV-2 was significantly higher in patients with ANA titre elevations (10).
How commonly these autoantibodies persist beyond the acute phase of SARS-CoV-2 infection and whether these long COVID patients with autoantibodies will transition to a classifiable autoimmune disease requires further research. However, case reports are emerging of COVID-19 patients with symptoms consistent with autoimmune diseases such as SLE (11), immune thrombocytopenic purpura (ITP) (12), Guillain-Barré syndrome (13), anti-phospholipid syndromes (APLS) (14) and multisystem inflammatory syndrome in children (MIS-C) (15).
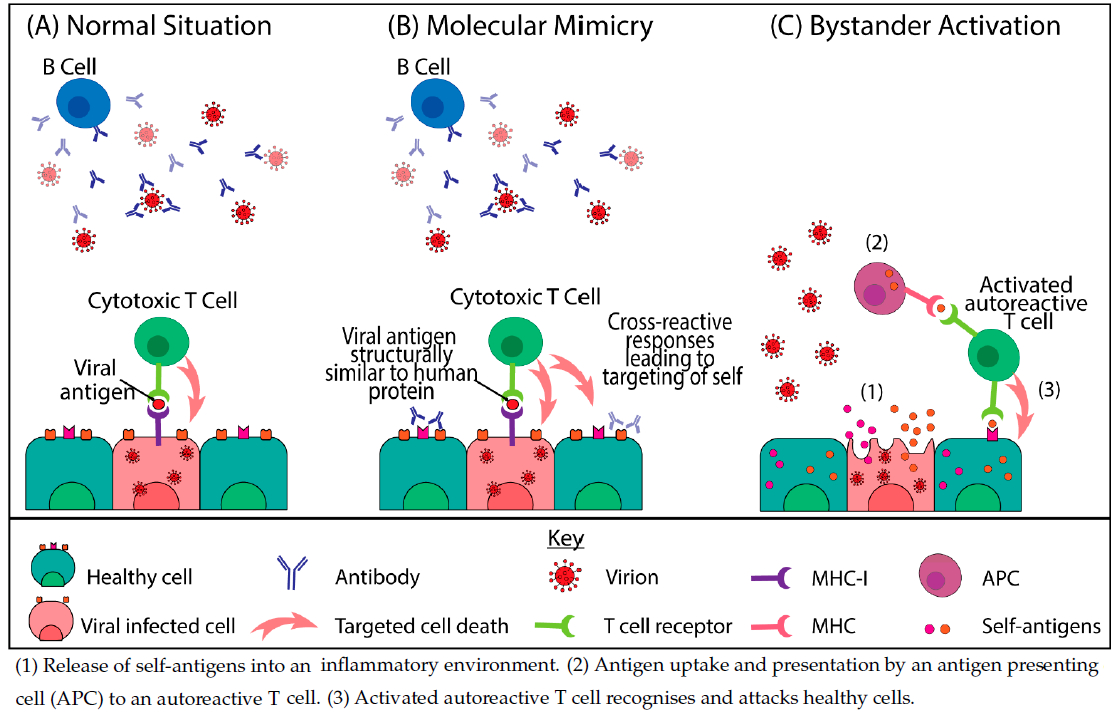
One hypothesis for the development of autoimmune pathologies from SARS-CoV-2 infection centres on the fact that the SARS-CoV-2 spike protein shares at least 30 epitopes with proteins in human organs, allowing the development of autoimmunity through cross-reactivity including molecular mimicry ![]() and/or bystander activation
and/or bystander activation ![]() (9,16). This molecular cross-reactivity results in the production of numerous autoantibodies and the activation of autoreactive T-cells that can cause tissue damage (17).
(9,16). This molecular cross-reactivity results in the production of numerous autoantibodies and the activation of autoreactive T-cells that can cause tissue damage (17).
Another possibility is that SARS-CoV-2 causes hyperactivation of the immune system. The SARS-CoV-2 spike protein contains sequence and structure motifs ![]() resembling those of a bacterial superantigen (staphylococcal enterotoxin B). Superantigens such as this can bind directly to T cell receptors and excessively activate immune cells, contributing to hyperinflammation and immune dysregulation (18).
resembling those of a bacterial superantigen (staphylococcal enterotoxin B). Superantigens such as this can bind directly to T cell receptors and excessively activate immune cells, contributing to hyperinflammation and immune dysregulation (18).
Reactivation of the Epstein-Barr virus (EBV), a human gamma-herpesvirus and one of the most ubiquitous viruses infecting up to 95% of the global population, is also linked to long COVID. The analysis of 185 COVID patients, in which 56 (30.3%) reported persisting long-COVID symptoms, found that 67.7% were positive for EBV reactivation compared to 10% of control subjects (19). EBV is a well-known viral trigger of autoimmunity in genetically susceptible individuals. After infection, it remains latent in B-cells in the body throughout life but can be periodically reactivated. EBV has been linked to autoimmune diseases including multiple sclerosis, SLE and RA (18,19,20).
Limitations and conclusion
The prospective study had several limitations, including a small sample size, the potential of recall bias of symptoms, and the inclusion of predominantly asymptomatic or mild COVID cases. In addition, the study was undertaken prior to the advent of vaccines, and future research needs to include vaccinated populations to determine if similar autoantibody responses are elicited.
Nevertheless, the study results are significant and demonstrate that SARS‑CoV‑2 infection, even in the absence of severe clinical disease, produces autoantibodies that persist for up to 6 months. Future research is required to understand the nature of triggered and persistent autoantibody activation among individuals exposed to SARS-CoV-2 and the effect on long-term clinical sequelae, including the potential to develop autoimmune disease.
Figure 1. Mechanisms of virally induced autoimmunity (23) CC BY 4.0