
Biofilms are communities of microorganisms encased in a self-produced extracellular polymer matrix attached to a living or non-living surface. They play a significant role in the persistence of bacterial infections. These highly complex microbial collections are widely distributed in the natural environment as well as in industrial, technological and medical settings. Biofilms are living and dynamic structures in perpetual alteration and are thought to be a major mode of bacterial survival for billions of years (1,2).
The biofilm matrix is made up of capsular polysaccharides which form a dense coat surrounding the bacterial cells, and exopolysaccharides that form a loose slime outside the cell. The biofilm matrix provides protection from detergents, antimicrobials, phagocytic cells and drying (3).
Figure 1. Schematic representation of biofilm stages
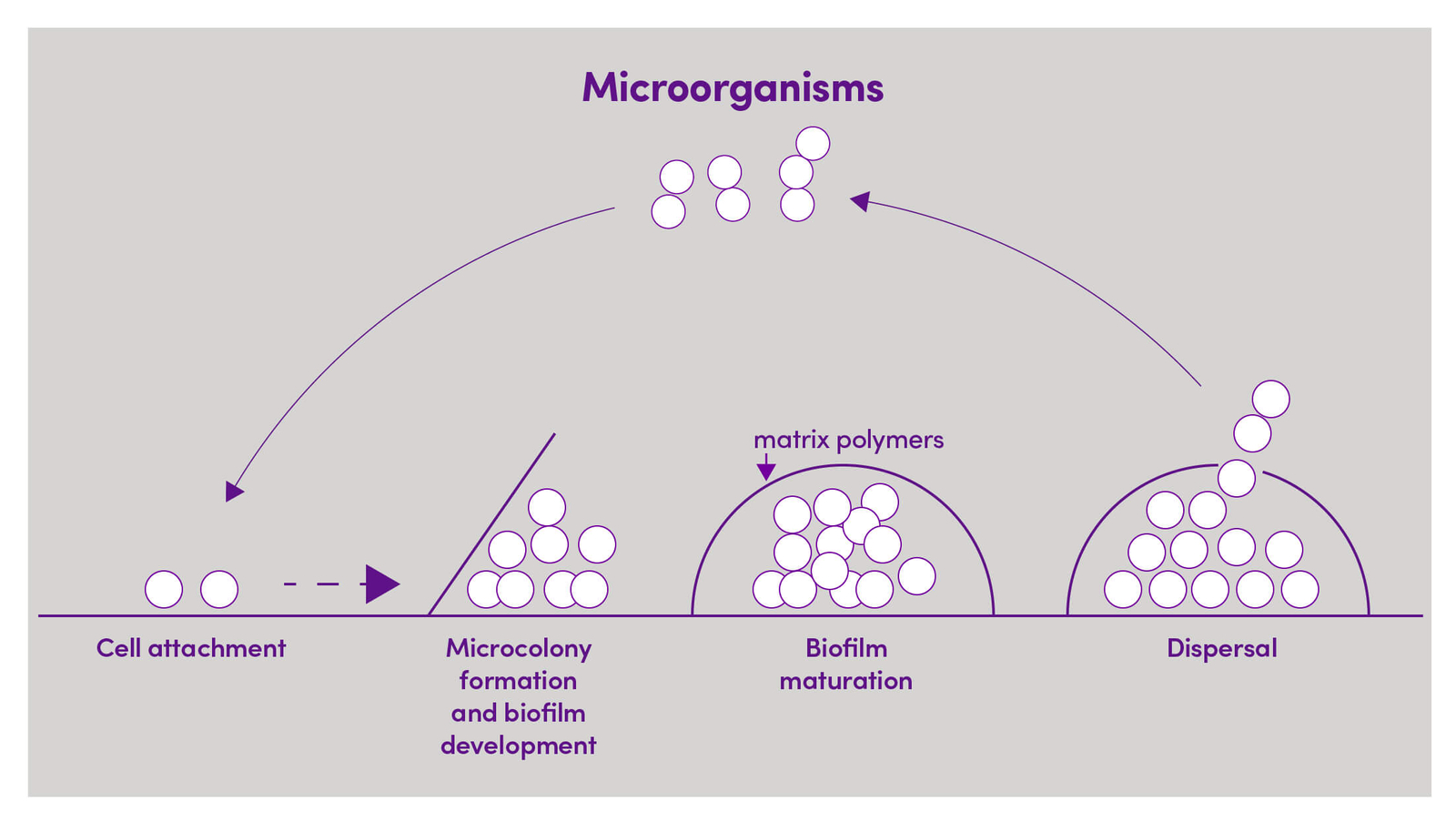
Bacterial biofilms are usually pathogenic in nature. It is estimated that 65% of all microbial infections and 80% of chronic infections are associated with biofilm formation. The diversity of biofilm-associated infections is increasing over time and its impact may be underestimated (4,5). Biofilm-associated infections include endocarditis, osteomyelitis, sinusitis, urinary tract infections, chronic prostatitis, periodontitis, chronic lung infection in cystic fibrosis patients, middle ear infections and various nosocomial ![]() infections, especially those related to implanted medical devices (see Table 1) (4).
infections, especially those related to implanted medical devices (see Table 1) (4).
Fungi also have the capacity to form biofilms. Candida albicans, a normal commensal on human mucosal surfaces and an opportunistic pathogen in immunocompromised patients, is the most clinically significant of the fungi species known to form biofilms (4).
Antibiotic and Immune System Resistance
Bacteria in biofilms can become resistant to both antibiotics and the host immune system, and tolerance to antimicrobial agents, biocides and antiseptics is a common feature of microbial biofilm formation. Biofilms act as diffusion barriers, allowing bacteria to continue multiplying inside the extracellular matrix up to a point at which they disperse to start forming new biofilm communities. Conventional antibiotics efficiently kill growing and dividing bacterial cells but are very inefficient at killing non-multiplying bacteria (i.e. ‘persisters’). A bactericidal antibiotic will kill the majority of cells in a biofilm, but persisters will survive. Once antibiotic treatment has ceased, persisters can revert to a growing state and repopulate the biofilm, causing a relapse of the infection. Some bacteria tolerate concentrations of antibiotics up to a thousand times higher than the minimum inhibitory concentration normally required for effective eradication (1,4,6).
The most effective and obvious ways of dealing with clinically important microbial biofilms are undoubtedly to inhibit their development.
Table 1. Diseases associated with biofilms (4,5,7,8,9,10,11,12,13)

Quorum Sensing and Quorum Sensing Interference
Quorum sensing (QS) is a form of bacterial cell-to-cell communication and is very important for the formation of bacterial biofilms. Quorum sensing regulates a number of activities in bacteria including pathogenicity and antibiotic resistance.
Quorum quenching (QQ) describes all processes that are involved in the disturbance of QS, leading to inhibition of bacterial biofilm formation. Naturally occurring QQ mechanisms act by blocking key steps of QS such as signal generation, signal accumulation and signal reception (5,14). Disruption of the bacterial QS systems occurs by naturally occurring quorum sensing inhibitory/interfering (QSI) molecules (14).
Figure 2. Quorum quenching mechanisms
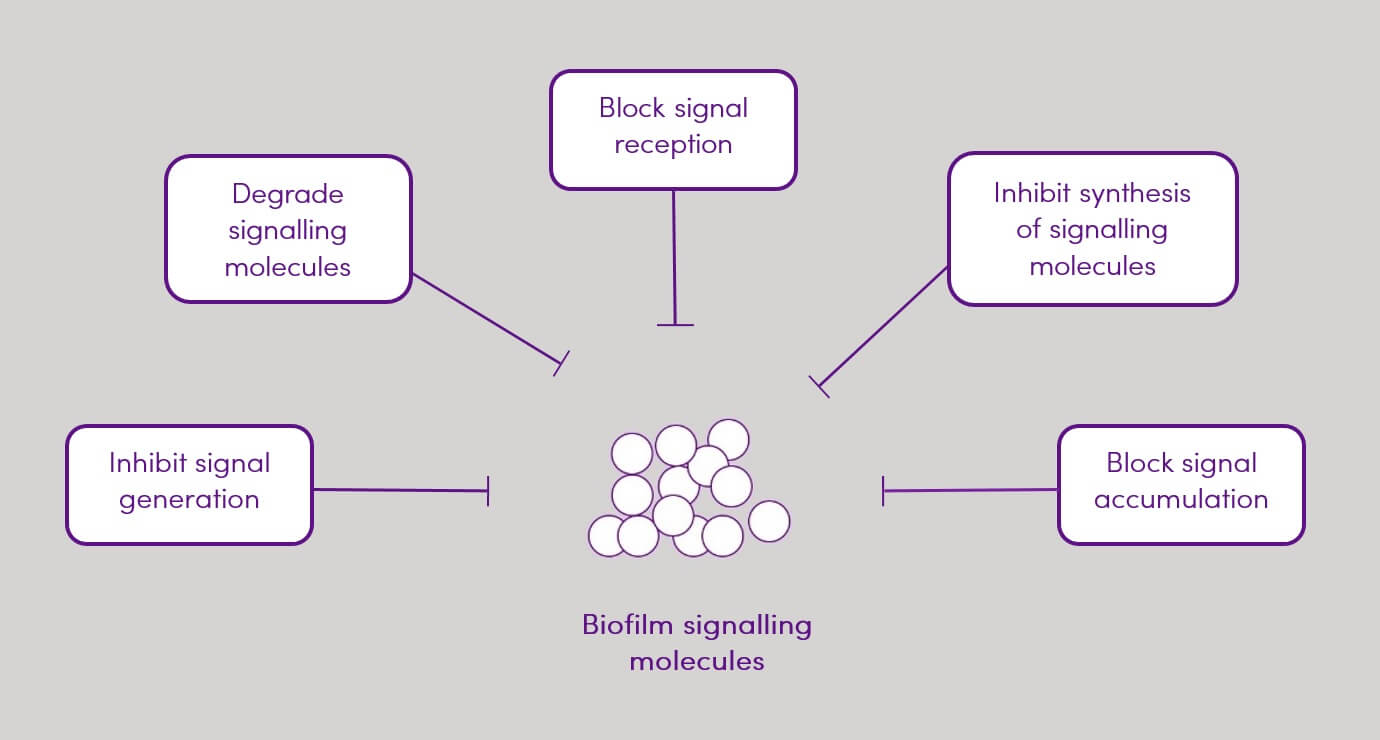
Plants have evolved to produce defence chemicals including QSI chemicals. Phytochemicals have been found to be promising alternatives to conventional antibiotic therapies for the control of bacterial infections due to their non-toxic nature and the similarity of their chemical structure to those of QS signals and/or their ability to degrade signal receptors. They may also entail less selective pressure and hence reduce the development of resistance. Currently, a large amount of research is being undertaken in screening medicinal plants for potential QSI activity (1,3,6).
The QSI activity of natural compounds is specific to their active components and specific bacterial strains. In other words, what works for one type of bacteria might not work for others. Confounding the research results further is the use of differing experimental models which might account for varying results across studies (1).
QSI Agents currently under investigation
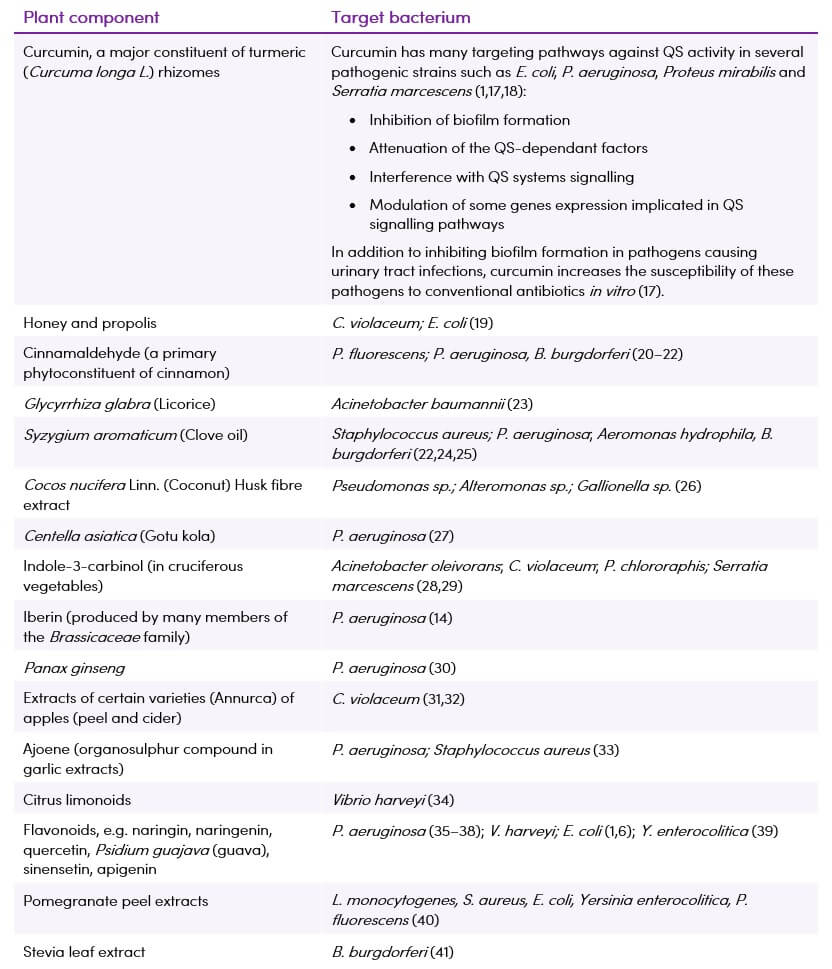
Medicinal plants contain several bio-active molecules such as terpenoids, polyphenols, flavonoids, tannins and anthocyanins, polyamines, cytokinins and polysaccharides that may be useful in counteracting bacteria resistance by targeting QS signalling pathways (1). Table 2 summarises some of the many plants/components that have been found to be useful in reducing biofilm formation in vitro. They may prove to be useful either alone or in combination with conventional antibiotics in the treatment of bacterial infections. Generally, plants exert better inhibition on gram-positive than on gram-negative bacteria ![]() (15).
(15).
Several spices such as turmeric, clove, black pepper and garlic have been used since ancient times to prevent gastrointestinal, pulmonary and urinary tract infections. They are known to produce a great spectrum of secondary metabolites many of which are found to have QSI properties (14).
Certain plant components (e.g. thymol, carvacrol and eugenol) enhance beneficial bacteria aggregation in addition to inhibiting biofilm activity (16).
Table 2. Some of the plant phytochemicals with in vitro quorum sensing interfering properties
Essential Oils
Among the QSI plant components under investigation, a great deal of attention is being paid to essential oils. Their effectiveness against a wide range of microorganisms is due to their ability to cause membrane destabilisation. Essential oils are lipophilic in nature which enables them to integrate into the lipids of the cell membrane and permeate the cell wall (42,43). Essential oils offer an advantage over other antimicrobial agents due to the fact that they offer broad antibacterial potency without the risk of bacterial resistance (44).
The antibacterial efficacy varies with oils as well as with different bacteria. For example (43):
- Sandalwood (Santalum album), manuka oil (Leptospermum scoparium) and vetiver (zizanioides) oils are highly active against gram-positive bacteria but have no activity against gram-negative bacteria
- Pseudomonas aeruginosa bacteria exhibit tolerance to inhibition by plant essential oils
- In some cases, a major constituent molecule is more effective than the essential oil itself, for example, carvacrol and eugenol from aromaticum (clove) oil or terpinen-4-ol in M. alternifolia (tea tree) oil
Various essential oils, when administered in combination with recommended antibiotics, can have an additive and synergistic effect and might be beneficial in difficult to treat wound infections (for example, basil, clary sage and rosemary oils). Other oils display greater antibacterial activity when used alone in treating wounds (for example, cinnamon, clove, thyme and lavender essential oils) (44).
Table 3. Essential oils displaying quorum sensing interfering properties

It is important to note that evidence to date relates primarily to in vitro studies and there are still a number of outstanding issues regarding the clinical relevance of current data (4,53,54):
- The anti-QS activity of medicinal plant extracts and essential oils is still poorly understood
- There is increasing evidence that in vitro biofilm assays may not accurately represent in vivo
- Host factors have many important implications for biofilm formation in the in vivo environment that are less appreciable in current in vitro
- Clinical trials are needed to confirm their relevance in human biofilms
N-Acetylcysteine (NAC)
Many in vitro studies have demonstrated that NAC is effective in inhibiting biofilm formation, disrupting preformed biofilms and reducing bacterial viability in biofilms (55,56). Results from clinical studies of NAC in respiratory tract infections have provided some encouraging findings that need to be confirmed.
NAC used alone or in combination with certain antibiotics can reduce biofilm formation by a variety of bacteria that are commonly isolated in hospitals, such as S. aureus, S. epidermidis, E. faecalis, Ps. aeruginosa, A. baumannii and K. pneumoniae (56).
Matrix degrading enzymes
Various enzymes, in combination with antibiotics, show effectiveness in eradication of biofilms in vitro (57). Examples include glycogen hydrolases, proteases, DNases and the fibrinolytic agent serrapeptase/serratiopeptidase (58,59,60,61). Nattokinase, a potent clot lysing enzyme initially isolated from Natto (a Japanese vegetable cheese) (62) is reported to disperse biofilms from infected central vein catheters in vivo (63).
Takeaway on Biofilm
- Biofilms are implicated in many persistent infections and are related to antibiotic and host immune system resistance
- Promising antibiotic alternatives based on medicinal plant compounds have been tested in vitro. The clinical viability has yet to be established (3)
- Different QSI agents have specific actions against different bacteria
- The use of essential oils with antibiotics can lead to a synergistic effect, or an antagonistic effect, depending on the plant and the bacteria. It is not a one size fits all approach (64)
- Outstanding clinical questions regarding QSI agents include (3):
- Do they have curative properties in addition to preventive properties?
- What are the most appropriate routes for delivery for practical applications?
- What is the impact on beneficial activities of the host-associated microbiota?
- Caution is required when ingesting approved essential oils. Always follow the manufacturer’s dosage and application guidelines
See Bo Hedgren's interview for more information on essential oils






