Chronic obstructive pulmonary disease (COPD) refers to a group of lung conditions, primarily emphysema ![]() and chronic bronchitis
and chronic bronchitis ![]() , which make breathing difficult. COPD is a multidimensional progressive inflammatory lung disease with pathological changes in the large and small airways (1,2). It is a lifelong condition involving irreversible lung damage, which is different from upper respiratory tract infections. It is characterised by increasing breathlessness, wheezing, chronic cough, and sputum production associated with irreversible, progressive inflammation and significant lung destruction with airflow limitation (3). A person with advanced COPD may be unable to perform daily tasks such as climbing stairs or cooking.
, which make breathing difficult. COPD is a multidimensional progressive inflammatory lung disease with pathological changes in the large and small airways (1,2). It is a lifelong condition involving irreversible lung damage, which is different from upper respiratory tract infections. It is characterised by increasing breathlessness, wheezing, chronic cough, and sputum production associated with irreversible, progressive inflammation and significant lung destruction with airflow limitation (3). A person with advanced COPD may be unable to perform daily tasks such as climbing stairs or cooking.
COPD is a common but poorly recognised disease, affecting over 400 million people worldwide (4). The exact pathogenesis of COPD remains largely unknown. Environmental risk factors such as cigarette smoking (including second-hand smoke exposure), bacterial or viral infection, and environmental pollution exposure can affect a range of potential lung function trajectories throughout life. Asthma symptoms may be part of COPD, and a history of asthma can increase the risk of developing the condition. Genetics may also play a role in developing COPD.
There is no cure for COPD, and early diagnosis and treatment from a multidisciplinary approach are critical for preventing or slowing progression and reducing mortality (5). Treatments include quitting smoking, oxygen therapy, medications that widen the airway, including nebulisers and inhalers and surgery.
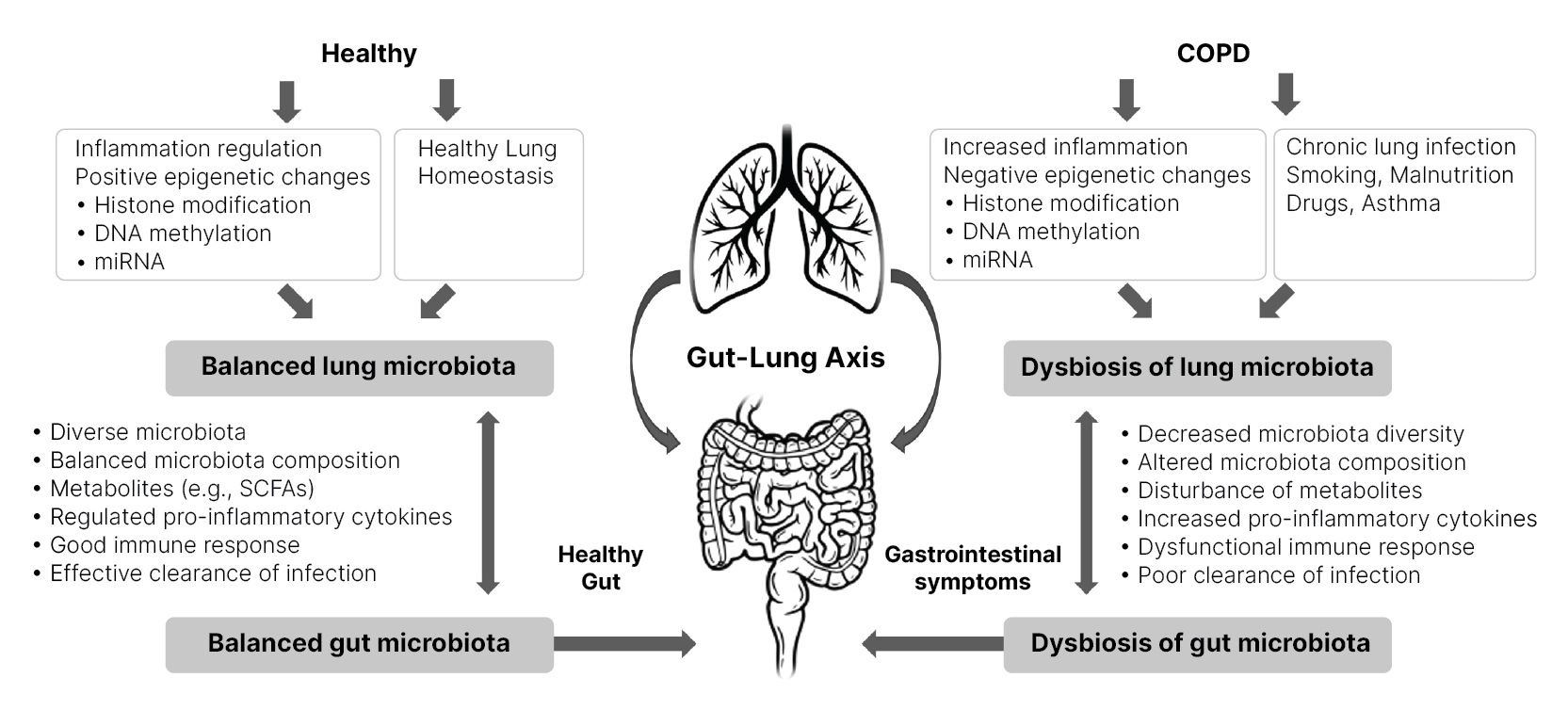
Disturbance in gut microbiota impacts multiple distant organs, including the lung. The gut-lung axis ![]() facilitates the passage of endotoxins, microbial metabolites, cytokines, and hormones into the bloodstream connecting the gut and lung, impacting immune response and homeostasis in the lungs and airway. In addition, changes in the intestinal microbiota can alter the composition of lung bacteria and vice versa (6,7,8,9). Therefore, the intestinal tract's bacteria can positively or negatively affect lung health.
facilitates the passage of endotoxins, microbial metabolites, cytokines, and hormones into the bloodstream connecting the gut and lung, impacting immune response and homeostasis in the lungs and airway. In addition, changes in the intestinal microbiota can alter the composition of lung bacteria and vice versa (6,7,8,9). Therefore, the intestinal tract's bacteria can positively or negatively affect lung health.
Although primarily considered a respiratory disease, COPD commonly co-occurs with chronic gastrointestinal (GI) tract diseases (9,10,11). Growing evidence indicates that dysbiosis of the gut microbiota is considered an important component in COPD pathophysiology (12,13,14,15,16). COPD is usually associated with decreased GI microbial diversity and immune system disturbance, contributing to chronic inflammation (5). In addition, increased GI permeability has been observed in patients with severe acute exacerbations of COPD (17) and altered gut microbiota is associated with COPD disease progression (18).
Mechanisms by which the microbiome affects COPD development and vice-versa (Figure 1):
- Regulation of the inflammatory environment.
- DNA methylation is affected by metabolites produced by gut bacteria (19) and is associated with gene expression profiles in COPD lung tissue (20).
- Histone modification - reduced histone enzymes in COPD may increase inflammation (21,22,23). The gut microbiome can modulate histone-producing enzyme activity by producing short-chain fatty acids (SCFAs) (24).
- The gut microbiome regulates microRNAs
 (miRNAs) (25), and miRNAs are capable of suppressing or preventing COPD development (26).
(miRNAs) (25), and miRNAs are capable of suppressing or preventing COPD development (26).
Figure 1 Gut-lung axis involvement in COPD. Adapted from (5) CC BY
Several risk factors, including smoking, diet, antibiotics, steroid treatments, and fibre intake affect the gut-lung axis ![]() in COPD.
in COPD.
Cigarette smoke exposure
Cigarette smoking is the most significant risk factor for COPD. Approximately 80% of COPD patients are past or current smokers (5). Tobacco smoke has a significant deleterious effect on lung tissue and the composition and relative abundance of the lung microbiome (27,28,29,30). Cigarette smoking has also been shown to reduce the diversity of the gut microbiome (5).
Dysbiosis in the gut can be modified through faecal transplantation in the cigarette-smoking-based model, indicating that gut microbiota may play a causal role in COPD pathogenesis (12).
Fibre, vitamin and folic acid intake
Individuals that consume less fibre, vitamins, and folic acid are prone to develop airflow limitations and COPD (31). The gut microbiome produces many metabolites, e.g. SCFAs, through fermentation of fibre. SCFAs are potent anti-inflammatory molecules. When absorbed into the systemic circulation, they can influence lung health. Dietary fibre leads to altered composition of gut and lung microbiota (32), and a lack of fermentable fibres can lead to malnourishment of the microbiota, resulting in gut dysbiosis and altered lung physiology (33,34).
Several studies indicate an inverse association between total and cereal dietary fibre intake and COPD incidence (35,36,37). In addition, preliminary evidence shows that dietary supplementation of probiotics Lactobacillus rhamnosus and Bifidobacterium breve prevents airway inflammation and lung damage in COPD mice (38).
Conclusion
While current management strategies prevent COPD exacerbation and relieve symptoms, new approaches are required to target the underlying disease process and reverse lung function deterioration. Gut dysbiosis in COPD may be a modifiable factor to be developed as a non-drug therapeutical strategy for preventing and managing the disease. Meanwhile, probiotic supplementation and faecal transplantation have also shown positive outcomes in experimental COPD models.