The Glymphatic System
The brain has long been thought to lack a lymphatic drainage system. In recent years, studies have discovered the glymphatic system, a macroscopic waste clearance system that utilizes a unique system of perivascular channels ![]() , formed by astroglial cells
, formed by astroglial cells ![]()
![]() , to promote efficient elimination of soluble proteins and metabolites from the central nervous system (1).
, to promote efficient elimination of soluble proteins and metabolites from the central nervous system (1).
Besides the clearance of cerebrospinal fluid (CSF) and interstitial fluid (ISF), the glymphatic system also facilitates the clearance of interstitial solutes such as amyloid-β ![]() and tau from the brain (2). The glymphatic system may also function to help distribute non-waste compounds, such as glucose, lipids, amino acids, and neurotransmitters related to volume transmission, in the brain (1).
and tau from the brain (2). The glymphatic system may also function to help distribute non-waste compounds, such as glucose, lipids, amino acids, and neurotransmitters related to volume transmission, in the brain (1).
The name "glymphatic system" was coined by the Danish neuroscientist Maiken Nedergaard in recognition of its dependence upon and the similarity of its functions to those of the peripheral lymphatic system. Even though the concept of the glymphatic system was developed only in recent years, many aspects of this highly organized pathway of CSF-ISF fluid exchange had already been described (1). The existence of the perivascular space was known but until very recently no specific function for it had been identified.
Studies have shown, the glymphatic system functions mainly during sleep and is largely disengaged during wakefulness (1). It is well documented that a multitude of biological events takes place during sleep (3). One of the functions of sleep appears to be that the glymphatic system is dramatically enhanced and that the brain clears itself of neurotoxic waste products produced during wakefulness (1). Slow-wave sleep seems to enhance the activity of the glymphatic system by approximately 60% (4).
Recent research has proposed low activity of the glymphatic system could be a major risk factor for the development of neurodegenerative diseases (1) and with a growing body of literature suggesting that sleep deprivation and sleep disorders can independently contribute to the development of cognitive impairment and dementia (5).
Proposed mechanism
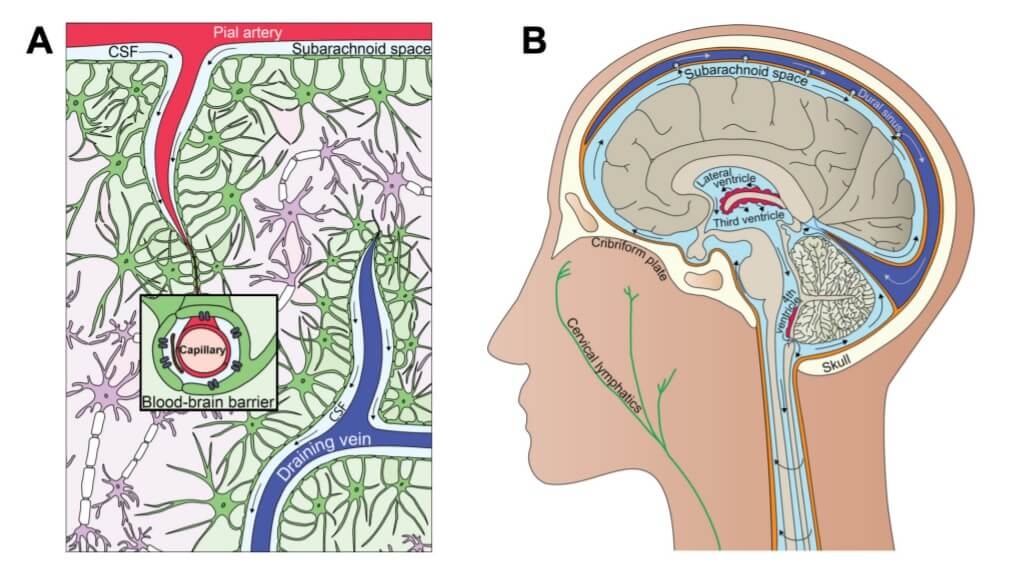
Cerebrospinal fluid (CSF) is continuously produced in the choroid plexus of the ventricles and transported into and out of the brain in part via the glymphatic pathway (6), a process which also facilitates removal of toxic waste proteins (6,7). See figure 1.
Soluble amyloid beta (Aβ) oligomers and tau protein have been shown to clear via the glymphatic pathway (7, 8) and it has been hypothesized therefore that dysfunction of glymphatic transport underlie buildup of extracellular Aβ aggregates and tau as seen in Alzheimer's disease (6,9).
Figure 1. Interrelationship between clearance and fluid systems of the brain (10) CC BY 4.0
A. The glymphatic system. B. The cerebrospinal fluid system.
Traditional understanding
Until recent years, there has been no evidence of a lymphatic system into the brain. The prevailing model was based on recycling: The brain clearing its own by-products of cellular function at an individual cell level.
With advanced microscopes and dyeing techniques, the brain’s interstitial space was mainly dedicated to physically removing the cell waste. The discovery of the glymphatic pathway was based on fluorescently tagged optical dyes of different molecular weights (MW) administered into CSF in combination with in vivo two-photon microscopy (4,7,9,11).
Current research findings
Traumatic brain injury
- The glymphatic system may be impaired after acute brain injuries such as ischemic stroke, intracranial haemorrhage or subarachnoid haemorrhage (1,12). Recent research has shown that the biomarkers of traumatic brain injury exit the brain via the glymphatic system (1)
Sleep disruption
- Recent research investigated whether clearance of amyloid-β
 (Ab) from the interstitial fluid varies with the brain state. Ab clearance was twice as fast during sleep and anaesthesia than during waking. Similar differences were found in the clearance of an inert tracer, suggesting that general removal of waste products is less efficient during waking (4)
(Ab) from the interstitial fluid varies with the brain state. Ab clearance was twice as fast during sleep and anaesthesia than during waking. Similar differences were found in the clearance of an inert tracer, suggesting that general removal of waste products is less efficient during waking (4)
- Brain energy metabolism only declines by 25% during sleep suggesting that sleep does not simply serve to conserve energy (13)
- The amount of sleep that constitutes “sufficient” sleep, however, continues to be debated. Nevertheless, it is generally agreed that people at the extremes of the sleep distribution, i.e., short (<5hrs) and long (>9 hrs) sleepers are subject to cognitive deficits and accelerated cognitive ageing (5)
Alzheimers
- Dysfunction of the glymphatic pathway may be implicated in the pathophysiology of Alzheimer's disease (9) and a variety of neurologic diseases, particularly those where an accumulation of pathologic solute is a prominent feature (14)
- It has been demonstrated that there is an age-associated decline in glymphatic CSF influx, as well as interstitial solute clearance, including Aβ, and this appears to be related to reduced penetrating arterial pulsatility
 in the aged brain (11,14)
in the aged brain (11,14)
Type 2 Diabetes
- Beyond clearance, this pathway has been shown to be critical for the distribution of nutrients, such as glucose, throughout the brain (15)
- In type II diabetes mellitus, an imbalance exists where there is increased glymphatic CSF influx without a concomitant increase in ISF efflux, thus leading to extracellular solute accumulation and cognitive decline (14, 16)
Future research
- The first studies of the glymphatic system in human subjects are expected to be published soon, as intrathecal
 administration of contrast agents is already approved for the study of CSF fluxes in patients undergoing treatment for spontaneous intracranial hypotension and CSF rhinorrhea
administration of contrast agents is already approved for the study of CSF fluxes in patients undergoing treatment for spontaneous intracranial hypotension and CSF rhinorrhea  (2, 17,18)
(2, 17,18)
- Future studies with a focus on the glymphatic system are expected to identify functions of CSF fluxes beyond removal of metabolic waste products. Researchers speculate that glymphatic influx provides an essential route for distribution of electrolytes, macromolecules, and other larger compounds that enter the brain predominantly via the blood-CSF barrier at the choroid plexus (1)
- The glymphatic system might serve as a path for the delivery and distribution of drugs including cancer drugs within the brain (1)
- Further researcher opportunities exist toward a mechanistic understanding of lymphatic and now glymphatic transport activity physiology, with a greater role for MR imaging and CSF flow measurements (2)
- Elevated levels of amyloid-β
 and tau can be detected in the CSF and blood of patients with mild forms of neurodegenerative diseases. Further testing may explore whether Osteopathic manipulative therapy could facilitate drainage of the interstitial soluble molecules and produce a measurable health benefit (2)
and tau can be detected in the CSF and blood of patients with mild forms of neurodegenerative diseases. Further testing may explore whether Osteopathic manipulative therapy could facilitate drainage of the interstitial soluble molecules and produce a measurable health benefit (2)
Conclusion
The glymphatic system is merely one component in a complex sequence of processes happening within the brain during sleep. It is becoming evident that sleep may play a crucial role in our brain’s physiological maintenance.
At present, there are no glymphatic-directed therapies to intervene in any of the mentioned various disease processes. As a result, the primary goal of future studies will be the identification of a novel target for up or down-regulating CSF-ISF exchange within the glymphatic pathway, ultimately to promote improved solute clearance in diseases where metabolite accumulation is a prominent feature (15).
More research is needed to resolve current discrepancies surrounding many other aspects of the system (19). To date, the concept of the glymphatic pathway as a waste removal system in the central nervous system has been strengthened considerably by recent preclinical research. There are still considerable gaps remaining with regard to its existence in the human brain. (3). Given the therapeutic potential that a comprehensive understanding of brain waste clearance pathways might offer, further research and clarification is greatly justified (20).